Nitric oxide regulates DELLA content and PIF expression to promote photomorphogenesis in Arabidopsis
- PMID: 21562334
- PMCID: PMC3135954
- DOI: 10.1104/pp.111.177741
Nitric oxide regulates DELLA content and PIF expression to promote photomorphogenesis in Arabidopsis
Abstract
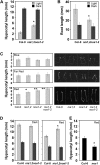
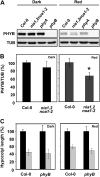
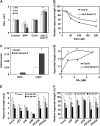
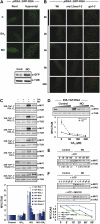
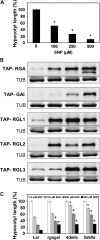
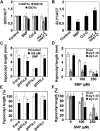
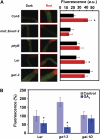
The transition from etiolated to green seedlings involves a shift from hypocotyl growth-promoting conditions to growth restraint. These changes occur through a complex light-driven process involving multiple and tightly coordinated hormonal signaling pathways. Nitric oxide (NO) has been lately characterized as a regulator of plant development interacting with hormone signaling. Here, we show that Arabidopsis (Arabidopsis thaliana) NO-deficient mutant hypocotyls are longer than those from wild-type seedlings under red light but not under blue or far-red light. Accordingly, exogenous treatment with the NO donor sodium nitroprusside and mutant plants with increased endogenous NO levels resulted in reduced hypocotyl length. In addition to increased hypocotyl elongation, NO deficiency led to increased anthocyanin levels and reduced PHYB content under red light, all processes governed by phytochrome-interacting factors (PIFs). NO-deficient plants accordingly showed an enhanced expression of PIF3, PIF1, and PIF4. Moreover, exogenous NO increased the levels of the gibberellin (GA)-regulated DELLA proteins and shortened hypocotyls, likely through the negative regulation of the GA Insensitive Dwarf1 (GID1)-Sleepy1 (SLY1) module. Consequently, NO-deficient seedlings displayed up-regulation of SLY1, defective DELLA accumulation, and altered GA sensitivity, thus resulting in defective deetiolation under red light. Accumulation of NO in wild-type seedlings undergoing red light-triggered deetiolation and elevated levels of NO in the GA-deficient ga1-3 mutant in darkness suggest a mutual NO-GA antagonism in controlling photomorphogenesis. PHYB-dependent NO production promotes photomorphogenesis by a GID1-GA-SLY1-mediated mechanism based on the coordinated repression of growth-promoting PIF genes and the increase in the content of DELLA proteins.
Figures

References
-
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94 - PubMed
-
- Achard P, Genschik P. (2009) Releasing the brakes of plant growth: how GAs shut down DELLA proteins. J Exp Bot 60: 1085–1092 - PubMed
-
- Achard P, Renou J-P, Berthomé R, Harberd NP, Genschik P. (2008) Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr Biol 18: 656–660 - PubMed
-
- Agrawal L, Chakraborty S, Jaiswal DK, Gupta S, Datta A, Chakraborty N. (2008) Comparative proteomics of tuber induction, development and maturation reveal the complexity of tuberization process in potato (Solanum tuberosum L.). J Proteome Res 7: 3803–3817 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

