Probing the interactions of an acyl carrier protein domain from the 6-deoxyerythronolide B synthase
- PMID: 21563224
- PMCID: PMC3149197
- DOI: 10.1002/pro.652
Probing the interactions of an acyl carrier protein domain from the 6-deoxyerythronolide B synthase
Abstract
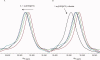
The assembly-line architecture of polyketide synthases (PKSs) provides an opportunity to rationally reprogram polyketide biosynthetic pathways to produce novel antibiotics. A fundamental challenge toward this goal is to identify the factors that control the unidirectional channeling of reactive biosynthetic intermediates through these enzymatic assembly lines. Within the catalytic cycle of every PKS module, the acyl carrier protein (ACP) first collaborates with the ketosynthase (KS) domain of the paired subunit in its own homodimeric module so as to elongate the growing polyketide chain and then with the KS domain of the next module to translocate the newly elongated polyketide chain. Using NMR spectroscopy, we investigated the features of a structurally characterized ACP domain of the 6-deoxyerythronolide B synthase that contribute to its association with its KS translocation partner. Not only were we able to visualize selective protein-protein interactions between the two partners, but also we detected a significant influence of the acyl chain substrate on this interaction. A novel reagent, CF₃-S-ACP, was developed as a ¹⁹F NMR spectroscopic probe of protein-protein interactions. The implications of our findings for understanding intermodular chain translocation are discussed.
Copyright © 2011 The Protein Society.
Figures


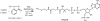


References
-
- Khosla C, Tang Y, Chen AY, Schnarr NA, Cane DE. Structure and mechanism of the 6-deoxyerythronolide B synthase. Annu Rev Biochem. 2007;76:195–221. - PubMed
-
- Weissman KJ, Leadlay PF. Combinatorial biosynthesis of reduced polyketides. Nat Rev Microbiol. 2005;3:925–936. - PubMed
-
- Fischbach MA, Walsh CT. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem Rev. 2006;106:3468–3496. - PubMed
-
- McDaniel R, Welch M, Hutchinson CR. Genetic approaches to polyketide antibiotics. Chem Rev. 2005;105:543–558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

