Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons
- PMID: 21564086
- PMCID: PMC3372824
- DOI: 10.1111/j.1476-5381.2011.01482.x
Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons
Abstract
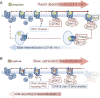
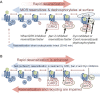
Agonists acting on µ-opioid receptors (MOR) are very effective analgesics but cause tolerance during long-term or repeated exposure. Intensive efforts have been made to find novel opioid agonists that are efficacious analgesics but can elude the signalling events that cause tolerance. µ-Opioid agonists differentially couple to downstream signalling mechanisms. Some agonists, such as enkephalins, D-Ala(2),N-Me-Phe(4),Gly(5)-ol]-enkephalin (DAMGO), methadone and sufentanyl are efficacious at mediating G-protein and effector coupling, as well as triggering MOR regulatory events that include MOR phosphorylation, β-arrestin binding, receptor endocytosis and recycling. By contrast, morphine and closely related alkaloids can mediate efficacious MOR-effector coupling but poorly trigger receptor regulation. Several models have been proposed to relate differential MOR regulation by different opioids with their propensity to cause tolerance. Most are based on dogma that β-arrestin-2 (βarr-2) binding causes MOR desensitization and/or that MOR endocytosis and recycling are required for receptor resensitization. This review will examine some of these notions in light of recent evidence establishing that MOR dephosphorylation and resensitization do not require endocytosis. Recent evidence from opioid-treated animals also suggests that impaired MOR-effector coupling is driven, at least in part, by enhanced desensitization, as well as impaired resensitization that appears to be βarr-2 dependent. Better understanding of how chronic exposure to opioids alters receptor regulatory mechanisms may facilitate the development of effective analgesics that produce limited tolerance.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures
References
-
- Adams JU, Paronis CA, Holtzman SG. Assessment of relative intrinsic activity of mu-opioid analgesics in vivo by using beta-funaltrexamine. J Pharmacol Exp Ther. 1990;255:1027–1032. - PubMed
-
- Arden JR, Segredo V, Wang Z, Lameh J, Sadee W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged µ-opioid receptor expressed in HEK 293 cells. J Neurochem. 1995;65:1636–1645. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

