Deconstructing the risk for malaria in United States donors deferred for travel to Mexico
- PMID: 21564102
- PMCID: PMC3645916
- DOI: 10.1111/j.1537-2995.2011.03158.x
Deconstructing the risk for malaria in United States donors deferred for travel to Mexico
Abstract
Background: More than 66,000 blood donors are deferred annually in the United States due to travel to malaria-endemic areas of Mexico. Mexico accounts for the largest share of malaria travel deferrals, yet it has extremely low risk for malaria transmission throughout most of its national territory, suggesting a suboptimal balance between blood safety and availability. This study sought to determine whether donor deferral requirements might be relaxed for parts of Mexico without compromising blood safety.
Study design and methods: Travel destination was recorded from a representative sample of presenting blood donors deferred for malaria travel from six blood centers during 2006. We imputed to these donors reporting Mexican travel a risk for acquiring malaria equivalent to Mexican residents in the destination location, adjusted for length of stay. We extrapolated these results to the overall US blood donor population.
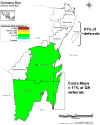
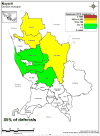
Results: Risk for malaria in Mexico varies significantly across endemic areas and is greatest in areas infrequently visited by study donors. More than 70% of blood donor deferrals were triggered by travel to the state of Quintana Roo on the Yucatán Peninsula, an area of very low malaria transmission. Eliminating the travel deferral requirement for all areas except the state of Oaxaca might result in the recovery of almost 65,000 blood donors annually at risk of approximately one contaminated unit collected every 20 years.
Conclusion: Deferral requirements should be relaxed for presenting donors who traveled to areas within Mexico that confer exceptionally small risks for malaria, such as Quintana Roo.
© 2011 American Association of Blood Banks.
Conflict of interest statement
The authors have no conflicts of interest or other financial involvement to declare.
Figures

References
-
- Mungai M, Tegtmeier G, Chamberland M, Parise M. Transfusion-transmitted malaria in the United States from 1963 through 1999. N Engl J Med. 2001;344:1973–8. - PubMed
-
- Parise ME. Traveler's malaria, locally-transmitted malaria and transfusion-transmitted malaria in the United States [monograph on the Internet] Rockville (MD): U.S. Food and Drug Administration, Center for Biologics Evaluation and Research (CBER), Food and Drug Administration; 2006. Available at http://www.fda.gov/BiologicsBloodVaccines/NewsEvents/WorkshopsMeetingsCo....
-
- Whitaker BI, Henry RA. The 2007 National Blood Collection and Utilization Survey Report. Washington (DC): Department of Health and Human Services; 2008.
-
- Zoon K. Memorandum to all registered blood establishments. Dept. of Health and Human Services, Food and Drug Administration; Jul 26, 1994. Recommendation for deferral of donors for malaria risk.
-
- Standards for blood banks and transfusion services. 26th. Bethesda, MD: AABB; 2009.
Publication types
MeSH terms
Grants and funding
- N01-HB-47172/HB/NHLBI NIH HHS/United States
- N01-HB-47171/HB/NHLBI NIH HHS/United States
- N01 HB057181/HL/NHLBI NIH HHS/United States
- N01 HB047172/HB/NHLBI NIH HHS/United States
- N01-HB-47181/HB/NHLBI NIH HHS/United States
- N01-HB-47170/HB/NHLBI NIH HHS/United States
- N01-HB-47175/HB/NHLBI NIH HHS/United States
- N01-HB-47174/HB/NHLBI NIH HHS/United States
- N01 HB047174/HB/NHLBI NIH HHS/United States
- N01 HB047170/HB/NHLBI NIH HHS/United States
- N01 HB047175/HB/NHLBI NIH HHS/United States
- N01 HB047169/HB/NHLBI NIH HHS/United States
- N01 HB047168/HB/NHLBI NIH HHS/United States
- N01-HB-47168/HB/NHLBI NIH HHS/United States
- N01 HB047171/HB/NHLBI NIH HHS/United States
- N01-HB-47169/HB/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

