Effects of safety warnings on prescription rates of cough and cold medicines in children below 2 years of age
- PMID: 21564162
- PMCID: PMC3099382
- DOI: 10.1111/j.1365-2125.2010.03860.x
Effects of safety warnings on prescription rates of cough and cold medicines in children below 2 years of age
Abstract
What is already known about this subject: • Cough and cold medicines are frequently used in children to treat upper respiratory tract infections without solid proof of benefits. • Safety issues have been raised about the use of these drugs in young children. • In 2007 international warnings were issued advising against use of these drugs in young children.
What this study adds: • Cough and cold medicines prescribing by primary care physicians has not really been influenced by international warnings in the Netherlands, where no additional national warnings were made and only partially in Italy. • A concerted action should be taken in Europe to advise strongly against the OTC use and prescription of cough and cold medicines in young children.
Aim: The aim of the study was to assess the influence of national and international warnings on the prescription rates of cough and cold medicines (CCMs) in the youngest children (<2 years) in the Netherlands and Italy.
Methods: Analysis of outpatient electronic medical records of children <2 years in Italy and the Netherlands was carried out. Age and country specific prescription prevalence rates were calculated for the period 2005-08. Comparisons of prescription rates in 2005 (pre) and 2008 (post) warnings were done by means of a chi-square test.
Results: The cohort consisted of 99,176 children <2 years of age. After international warnings, overall prescription rates for CCMs decreased slightly from 83 to 77/1000 person years (P= 0.05) in Italy and increased in the Netherlands from 74 to 92/1000 children per year. Despite the international warnings, prescription rates for nasal sympathomimetics and opium alkaloids increased in the Netherlands (P < 0.01). In Italy a significant decrease in the prescription rates of opium alkaloids and other cough suppressants (P < 0.01) was observed, and also a significant reduction in use of combinations of nasal sympathomimetics.
Conclusion: Despite the international safety warnings and negative benefit-risk profiles, prescription rates of cough and cold medicines remain substantial and were hardly affected by the warnings, especially in the Netherlands where no warning was issued. The hazards of use of these medicines in young children should be explicitly stipulated by the European Medicines Agency and all national agencies, in order to increase awareness amongst physicians and caretakers and reduce heterogeneity across the EU.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures

 ); IPCI 95% CI upper limit (
); IPCI 95% CI upper limit ( ); IPCI 95% CI lower limit (
); IPCI 95% CI lower limit ( ); Pedianet (
); Pedianet ( ); Pedianet 95% CI upper limit (
); Pedianet 95% CI upper limit ( ); Pedianet 95% CI lower limit (
); Pedianet 95% CI lower limit ( )
)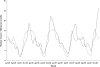
 ); Pedianet (
); Pedianet ( )
)Similar articles
-
Cold preparation use in young children after FDA warnings: do concerns still exist?Clin Pediatr (Phila). 2013 Jun;52(6):534-9. doi: 10.1177/0009922813482761. Epub 2013 Mar 28. Clin Pediatr (Phila). 2013. PMID: 23539689
-
Debate continues over the safety of cold and cough medicines for children.JAMA. 2008 Nov 26;300(20):2354-6. doi: 10.1001/jama.2008.647. JAMA. 2008. PMID: 19033577 No abstract available.
-
The impact of pediatric labeling changes on prescribing patterns of cough and cold medications.J Pediatr. 2014 Nov;165(5):1024-8.e1. doi: 10.1016/j.jpeds.2014.07.047. Epub 2014 Sep 5. J Pediatr. 2014. PMID: 25195159
-
Safety and efficacy of over-the-counter cough and cold medicines for use in children.Expert Opin Drug Saf. 2010 Mar;9(2):233-42. doi: 10.1517/14740330903496410. Expert Opin Drug Saf. 2010. PMID: 20001764 Review.
-
Over-the-counter cough and cold medication use in young children.Pediatr Nurs. 2008 Mar-Apr;34(2):174-80, 184. Pediatr Nurs. 2008. PMID: 18543844 Review.
Cited by
-
The common cold: potential for future prevention or cure.Curr Allergy Asthma Rep. 2014 Feb;14(2):413. doi: 10.1007/s11882-013-0413-5. Curr Allergy Asthma Rep. 2014. PMID: 24415465 Free PMC article. Review.
-
Pediatrician's cough and cold medication prescription for hypothetical cases - A cross-sectional multi-centric study.Saudi Pharm J. 2016 Mar;24(2):176-81. doi: 10.1016/j.jsps.2015.02.011. Epub 2015 Mar 19. Saudi Pharm J. 2016. PMID: 27013910 Free PMC article.
-
A Review of the Ingredients Contained in Over the Counter (OTC) Cough Syrup Formulations in Kenya. Are They Harmful to Infants?PLoS One. 2015 Nov 5;10(11):e0142092. doi: 10.1371/journal.pone.0142092. eCollection 2015. PLoS One. 2015. PMID: 26540251 Free PMC article.
-
Noscapine, an Emerging Medication for Different Diseases: A Mechanistic Review.Evid Based Complement Alternat Med. 2021 Nov 29;2021:8402517. doi: 10.1155/2021/8402517. eCollection 2021. Evid Based Complement Alternat Med. 2021. Retraction in: Evid Based Complement Alternat Med. 2023 Dec 13;2023:9796340. doi: 10.1155/2023/9796340. PMID: 34880922 Free PMC article. Retracted. Review.
-
Noscapine, a possible drug candidate for attenuation of cytokine release associated with SARS-CoV-2.Drug Dev Res. 2020 Nov;81(7):765-767. doi: 10.1002/ddr.21676. Epub 2020 Apr 26. Drug Dev Res. 2020. PMID: 32337769 Free PMC article.
References
-
- Sharfstein JM, North M, Serwint JR. Over the counter but no longer under the radar – pediatric cough and cold medications. N Engl J Med. 2007;357:2321–4. - PubMed
-
- FDA. FDA Recommends that Over-the-Counter (OTC) Cough and Cold Products not be used for Infants and Children under 2 Years of Age. 2008. Available at: http://www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm051137.html (last accessed 3 October 2009)
-
- MHRA. Press release: better medicines for children's coughs and colds. 2009. Available at: http://www.mhra.gov.uk/NewsCentre/Pressreleases/CON038902 (last accessed 3 October 2009)
-
- Canada H. Health Canada Releases Decision on the Labelling of Cough and Cold Products for Children. 2008. Available at: http://www.hc-sc.gc.ca/ahc-asc/media/advisories-avis/_2008/2008_184-eng.php (last accessed 3 October 2009)
-
- NPR/Kaiser Family Foundation/Harvard School of Public Health. Children's OTC cold medicines: the public, and parents, weigh in 2007. Available at: http://www.kff.org/kaiserpolls/upload/7725.pdf (last accessed 5 October 2009)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

