The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes
- PMID: 21565531
- PMCID: PMC3103637
- DOI: 10.1016/j.immuni.2011.04.013
The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes
Abstract
The intracellular sensor Nod2 is activated in response to bacteria, and the impairment of this response is linked to Crohn's disease. However, the function of Nod2 in host defense remains poorly understood. We found that Nod2-/- mice exhibited impaired intestinal clearance of Citrobacter rodentium, an enteric bacterium that models human infection by pathogenic Escherichia coli. The increased bacterial burden was preceded by reduced CCL2 chemokine production, inflammatory monocyte recruitment, and Th1 cell responses in the intestine. Colonic stromal cells, but not epithelial cells or resident CD11b+ phagocytic cells, produced CCL2 in response to C. rodentium in a Nod2-dependent manner. Unlike resident phagocytic cells, inflammatory monocytes produced IL-12, a cytokine that induces adaptive immunity required for pathogen clearance. Adoptive transfer of Ly6C(hi) monocytes restored the clearance of the pathogen in infected Ccr2-/- mice. Thus, Nod2 mediates CCL2-CCR2-dependent recruitment of inflammatory monocytes, which is important in promoting bacterial eradication in the intestine.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures

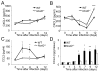

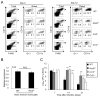
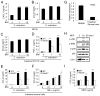
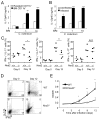
Comment in
-
Gut Nod2 calls the bone marrow for monocyte reinforcements.Immunity. 2011 May 27;34(5):693-5. doi: 10.1016/j.immuni.2011.04.016. Immunity. 2011. PMID: 21616438
References
-
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. - PubMed
-
- Borenshtein D, McBee ME, Schauer DB. Utility of the Citrobacter rodentium infection model in laboratory mice. Curr Opin Gastroenterol. 2008;24:32–37. - PubMed
-
- Bry L, Brenner MB. Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J Immunol. 2004;172:433–441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

