Local interstitial delivery of z-butylidenephthalide by polymer wafers against malignant human gliomas
- PMID: 21565841
- PMCID: PMC3107093
- DOI: 10.1093/neuonc/nor021
Local interstitial delivery of z-butylidenephthalide by polymer wafers against malignant human gliomas
Abstract
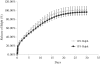
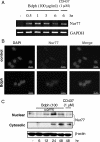
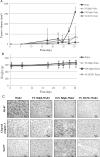
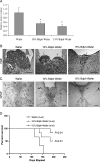
We have shown that the natural compound z-butylidenephthalide (Bdph), isolated from the chloroform extract of Angelica sinensis, has antitumor effects. Because of the limitation of the blood-brain barrier, the Bdph dosage required for treatment of glioma is relatively high. To solve this problem, we developed a local-release system with Bdph incorporated into a biodegradable polyanhydride material, p(CPP-SA; Bdph-Wafer), and investigated its antitumor effects. On the basis of in vitro release kinetics, we demonstrated that the Bdph-Wafer released 50% of the available Bdph by the sixth day, and the release reached a plateau phase (90% of Bdph) by the 30th day. To investigate the in situ antitumor effects of the Bdph-Wafer on glioblastoma multiforme (GBM), we used 2 xenograft animal models-F344 rats (for rat GBM) and nude mice (for human GBM)-which were injected with RG2 and DBTRG-05MG cells, respectively, for tumor formation and subsequently treated subcutaneously with Bdph-Wafers. We observed a significant inhibitory effect on tumor growth, with no significant adverse effects on the rodents. Moreover, we demonstrated that the antitumor effect of Bdph on RG2 cells was via the PKC pathway, which upregulated Nurr77 and promoted its translocation from the nucleus to the cytoplasm. Finally, to study the effect of the interstitial administration of Bdph in cranial brain tumor, Bdph-Wafers were surgically placed in FGF-SV40 transgenic mice. Our Bdph-Wafer significantly reduced tumor size in a dose-dependent manner. In summary, our study showed that p(CPP-SA) containing Bdph delivered a sufficient concentration of Bdph to the tumor site and effectively inhibited the tumor growth in the glioma.
Figures




References
-
- Santarius T, Kirsch M, Rossi ML, Black PM. Molecular aspects of neuro-oncology. Clin Neurol Neurosur. 1997;99:184–195. doi:10.1016/S0303-8467(97)00025-5. - DOI - PubMed
-
- Cobb MA, Husain M, Andersen BJ, al-Mefty O. Significance of proliferating cell nuclear antigen in predicting recurrence of intracranial meningioma. J Neurosurg. 1996;84:85–90. doi:10.3171/jns.1996.84.1.0085. - DOI - PubMed
-
- Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi:10.1200/JCO.2003.05.063. - DOI - PubMed
-
- Blacklock JB, Wright DC, Dedrick RL, et al. Drug streaming during intra-arterial chemotherapy. J Neurosurg. 1986;64:284–291. doi:10.3171/jns.1986.64.2.0284. - DOI - PubMed
-
- Shapiro WR, Green SB. Reevaluating the efficacy of intra-arterial BCNU. J Neurosurg. 1987;66:313–315. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

