Resource partitioning to male and female flowers of Spinacia oleracea L. in relation to whole-plant monocarpic senescence
- PMID: 21565983
- PMCID: PMC3153683
- DOI: 10.1093/jxb/err148
Resource partitioning to male and female flowers of Spinacia oleracea L. in relation to whole-plant monocarpic senescence
Abstract
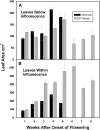
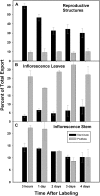
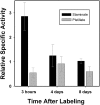
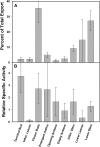
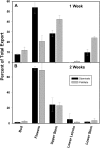
Male plants of spinach (Spinacea oleracea L.) senesce following flowering. It has been suggested that nutrient drain by male flowers is insufficient to trigger senescence. The partitioning of radiolabelled photosynthate between vegetative and reproductive tissue was compared in male (staminate) versus female (pistillate) plants. After the start of flowering staminate plants senesce 3 weeks earlier than pistillate plants. Soon after the start of flowering, staminate plants allocated several times as much photosynthate to flowering structures as did pistillate plants. The buds of staminate flowers with developing pollen had the greatest draw of photosynthate. When the staminate plants begin to show senescence 68% of fixed C was allocated to the staminate reproductive structures. In the pistillate plants, export to the developing fruits and young flowers remained near 10% until mid-reproductive development, when it increased to 40%, declining to 27% as the plants started to senesce. These differences were also present on a sink-mass corrected basis. Flowers on staminate spinach plants develop faster than pistillate flowers and have a greater draw of photosynthate than do pistillate flowers and fruits, although for a shorter period. Pistillate plants also produce more leaf area within the inflorescence to sustain the developing fruits. The (14)C in the staminate flowers declined due to respiration, especially during pollen maturation; no such loss occurred in pistillate reproductive structures. The partitioning to the reproductive structures correlates with the greater production of floral versus vegetative tissue in staminate plants and their more rapid senescence. As at senescence the leaves still had adequate carbohydrate, the resources are clearly phloem-transported compounds other than carbohydrates. The extent of the resource redistribution to reproductive structures and away from the development of new vegetative sinks, starting very early in the reproductive phase, is sufficient to account for the triggering of senescence in the rest of the plant.
Figures


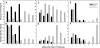



Similar articles
-
Experimental defoliation affects male but not female reproductive performance of the tropical monoecious plant Croton suberosus (Euphorbiaceae).Ann Bot. 2010 Aug;106(2):359-69. doi: 10.1093/aob/mcq117. Epub 2010 Jun 2. Ann Bot. 2010. PMID: 20519239 Free PMC article.
-
Preformation and distribution of staminate and pistillate flowers in growth units of Nothofagus alpina and N. obliqua (Nothofagaceae).Ann Bot. 2009 Feb;103(3):411-21. doi: 10.1093/aob/mcn235. Epub 2008 Nov 25. Ann Bot. 2009. PMID: 19033286 Free PMC article.
-
Pollination biology in the dioecious orchid Catasetum uncatum: How does floral scent influence the behaviour of pollinators?Phytochemistry. 2015 Aug;116:149-161. doi: 10.1016/j.phytochem.2015.02.027. Epub 2015 Mar 11. Phytochemistry. 2015. PMID: 25771507
-
Sugars and flowering in the grapevine (Vitis vinifera L.).J Exp Bot. 2008;59(10):2565-78. doi: 10.1093/jxb/ern135. Epub 2008 May 28. J Exp Bot. 2008. PMID: 18508810 Review.
-
Photosynthetic activity of reproductive organs.J Exp Bot. 2019 Mar 27;70(6):1737-1754. doi: 10.1093/jxb/erz033. J Exp Bot. 2019. PMID: 30824936 Review.
Cited by
-
Metabolic and transcriptional transitions in barley glumes reveal a role as transitory resource buffers during endosperm filling.J Exp Bot. 2015 Mar;66(5):1397-411. doi: 10.1093/jxb/eru492. Epub 2015 Jan 22. J Exp Bot. 2015. PMID: 25617470 Free PMC article.
-
The effect of injury on whole-plant senescence: an experiment with two root-sprouting Barbarea species.Ann Bot. 2016 Apr;117(4):667-79. doi: 10.1093/aob/mcw010. Epub 2016 Mar 14. Ann Bot. 2016. PMID: 26975314 Free PMC article.
-
The "STAY-GREEN" trait and phytohormone signaling networks in plants under heat stress.Plant Cell Rep. 2017 Jul;36(7):1009-1025. doi: 10.1007/s00299-017-2119-y. Epub 2017 May 8. Plant Cell Rep. 2017. PMID: 28484792 Review.
-
Hormonal regulation of leaf senescence through integration of developmental and stress signals.Plant Mol Biol. 2013 Aug;82(6):547-61. doi: 10.1007/s11103-013-0043-2. Epub 2013 Mar 16. Plant Mol Biol. 2013. PMID: 23504405 Review.
-
Overexpression of the maize Corngrass1 microRNA prevents flowering, improves digestibility, and increases starch content of switchgrass.Proc Natl Acad Sci U S A. 2011 Oct 18;108(42):17550-5. doi: 10.1073/pnas.1113971108. Epub 2011 Oct 10. Proc Natl Acad Sci U S A. 2011. PMID: 21987797 Free PMC article.
References
-
- Borras L, Maddonni GA, Otegui ME. Leaf senescence in maize hybrids: plant population, row spacing and kernel set effects. Field Crops Research. 2003;82:13–26.
-
- Borrell A, Hammer G, Van Oosterom E. Stay-green: a consequence of the balance between supply and demand for nitrogen during grain filling? Annals of Applied Biology. 2001;138:91–95.
-
- Cairns AJ. Colorimetric microtiter plate assay of glucose and fructose by enzyme-linked formazan production applicability to the measurement of fructosyltransferase activity in higher plants. Analytical Biochemistry. 1987;167:270–278. - PubMed
-
- Crafts-Brandner SJ, Egli DB. Modification of seed growth in soybean by physical restraint effect on leaf senescence. Journal of Experimental Botany. 1987;38:2043–2049.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

