Decreased specific force and power production of muscle fibers from myostatin-deficient mice are associated with a suppression of protein degradation
- PMID: 21565991
- PMCID: PMC3137541
- DOI: 10.1152/japplphysiol.00126.2011
Decreased specific force and power production of muscle fibers from myostatin-deficient mice are associated with a suppression of protein degradation
Erratum in
- J Appl Physiol. 2011 Dec;111(6):1889
Abstract
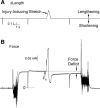
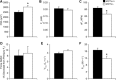
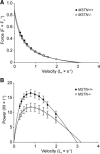
Myostatin (MSTN) is a member of the transforming growth factor-β superfamily of cytokines and is a negative regulator of skeletal muscle mass. Compared with MSTN(+/+) mice, the extensor digitorum longus muscles of MSTN(-/-) mice exhibit hypertrophy, hyperplasia, and greater maximum isometric force production (F(o)), but decreased specific maximum isometric force (sF(o); F(o) normalized by muscle cross-sectional area). The reason for the reduction in sF(o) was not known. Studies in myotubes indicate that inhibiting myostatin may increase muscle mass by decreasing the expression of the E3 ubiquitin ligase atrogin-1, which could impact the force-generating capacity and size of muscle fibers. To gain a greater understanding of the influence of myostatin on muscle contractility, we determined the impact of myostatin deficiency on the contractility of permeabilized muscle fibers and on the levels of atrogin-1 and ubiquitinated myosin heavy chain in whole muscle. We hypothesized that single fibers from MSTN(-/-) mice have a greater F(o), but no difference in sF(o), and a decrease in atrogin-1 and ubiquitin-tagged myosin heavy chain levels. The results indicated that fibers from MSTN(-/-) mice have a greater cross-sectional area, but do not have a greater F(o) and have a sF(o) that is significantly lower than fibers from MSTN(+/+) mice. The extensor digitorum longus muscles from MSTN(-/-) mice also have reduced levels of atrogin-1 and ubiquitinated myosin heavy chain. These findings suggest that myostatin inhibition in otherwise healthy muscle increases the size of muscle fibers and decreases atrogin-1 levels, but does not increase the force production of individual muscle fibers.
Figures

References
-
- Allen DL, Unterman TG. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol 292: C188–C199, 2007 - PubMed
-
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 - PubMed
-
- Bogdanovich S, Perkins KJ, Krag TOB, Whittemore LA, Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J 19: 543–549, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

