Anti-phospholipase A2 receptor antibody in membranous nephropathy
- PMID: 21566055
- PMCID: PMC3103733
- DOI: 10.1681/ASN.2010090967
Anti-phospholipase A2 receptor antibody in membranous nephropathy
Abstract
The M-type phospholipase A2 receptor (PLA2R) is a target autoantigen in adult idiopathic membranous nephropathy (MN), but the prevalence of autoantibodies against PLA2R is unknown among Chinese patients with MN. Here, we measured anti-PLA2R antibody in the serum of 60 patients with idiopathic MN, 20 with lupus-associated MN, 16 with hepatitis B (HBV)-associated MN, and 10 with tumor-associated MN. Among patients with idiopathic MN, 49 (82%) had detectable anti-PLA2R autoantibodies using a Western blot assay; an assay with greater sensitivity detected very low titers of anti-PLA2R in 10 of the remaining 11 patients. Using the standard assay, we detected anti-PLA2R antibody in only 1 patient with lupus, 1 with HBV, and 3 with cancer, producing an overall specificity of 89% in this cohort limited to patients with secondary MN. The enhanced assay detected low titers of anti-PLA2R in only 2 additional samples of HBV-associated MN. In summary, these results suggest that PLA2R is a major target antigen in Chinese idiopathic MN and that detection of anti-PLA2R is a sensitive test for idiopathic MN.
Figures
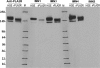

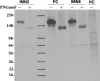



References
-
- Wasserstein AG: Membranous glomerulonephritis. J Am Soc Nephrol 8: 664–674, 1997 - PubMed
-
- Li LS, Liu ZH: Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int 66: 920–923, 2004 - PubMed
-
- Kerjaschki D: Pathomechanisms and molecular basis of membranous nephropathy. Lancet 364: 1194–1196, 2004 - PubMed
-
- Salant DJ: In search of the elusive membranous nephropathy antigen. Nephron Physiol 112: 11–12, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

