The spectrin-based membrane skeleton stabilizes mouse megakaryocyte membrane systems and is essential for proplatelet and platelet formation
- PMID: 21566095
- PMCID: PMC3156050
- DOI: 10.1182/blood-2011-01-330688
The spectrin-based membrane skeleton stabilizes mouse megakaryocyte membrane systems and is essential for proplatelet and platelet formation
Abstract
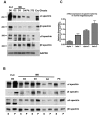
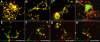
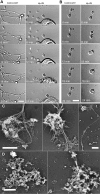
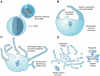
Megakaryocytes generate platelets by remodeling their cytoplasm first into proplatelets and then into preplatelets, which undergo fission to generate platelets. Although the functions of microtubules and actin during platelet biogenesis have been defined, the role of the spectrin cytoskeleton is unknown. We investigated the function of the spectrin-based membrane skeleton in proplatelet and platelet production in murine megakaryocytes. Electron microscopy revealed that, like circulating platelets, proplatelets have a dense membrane skeleton, the main fibrous component of which is spectrin. Unlike other cells, megakaryocytes and their progeny express both erythroid and nonerythroid spectrins. Assembly of spectrin into tetramers is required for invaginated membrane system maturation and proplatelet extension, because expression of a spectrin tetramer-disrupting construct in megakaryocytes inhibits both processes. Incorporation of this spectrin-disrupting fragment into a novel permeabilized proplatelet system rapidly destabilizes proplatelets, causing blebbing and swelling. Spectrin tetramers also stabilize the "barbell shapes" of the penultimate stage in platelet production, because addition of the tetramer-disrupting construct converts these barbell shapes to spheres, demonstrating that membrane skeletal continuity maintains the elongated, pre-fission shape. The results of this study provide evidence for a role for spectrin in different steps of megakaryocyte development through its participation in the formation of invaginated membranes and in the maintenance of proplatelet structure.
Figures



Comment in
-
Proplatelet formation flex required.Blood. 2011 Aug 11;118(6):1434-5. doi: 10.1182/blood-2011-05-355180. Blood. 2011. PMID: 21835962 No abstract available.
References
-
- Fox J, Reynolds C, Morrow J, Phillips D. Spectrin is associated with membrane-bound actin filaments in platelets and is hydrolyzed by the Ca2+-dependent protease during platelet activation. Blood. 1987;69(2):537–545. - PubMed
-
- Fox J, Aggerbeck L, Berndt M. Structure of the glycoprotein Ib-IX complex from platelet membranes. J Biol Chem. 1988;263(10):4882–4890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

