Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial
- PMID: 21566104
- PMCID: PMC3109926
- DOI: 10.2215/CJN.09421010
Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial
Abstract
Background and objectives: Prednisone and calcineurin inhibitors are the mainstay therapy of idiopathic nephrotic syndrome (INS) in children. However, drug dependence and toxicity associated with protracted use are common. Case series suggest that the anti-CD20 monoclonal antibody rituximab (RTX) may maintain disease remission.
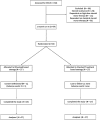
Design, setting, participants, & measurements: This open-label randomized controlled trial was powered to show that a strategy based on RTX and lower doses of prednisone and calcineurin inhibitors was noninferior to standard doses of these agents in maintaining 3-month proteinuria as low as baseline or up to 1 g/d greater (noninferiority margin). Participants were stratified by the presence of toxicity to prednisone/calcineurin inhibitors and centrally assigned to add RTX (Mabthera, 375 mg/m(2) intravenously) to lower doses of standard agents or to continue with current therapy alone. The risk of relapse was a secondary outcome.
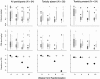
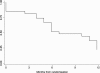
Results: Fifty-four children (mean age 11 ± 4 years) with INS dependent on prednisone and calcineurin inhibitors for >12 months were randomized. Three-month proteinuria was 70% lower in the RTX arm (95% confidence interval 35% to 86%) as compared with standard therapy arm (intention-to-treat); relapse rates were 18.5% (intervention) and 48.1% (standard arm) (P = 0.029). Probabilities of being drug-free at 3 months were 62.9% and 3.7%, respectively (P < 0.001); 50% of RTX cases were in stable remission without drugs after 9 months.
Conclusions: Rituximab and lower doses of prednisone and calcineurin inhibitors are noninferior to standard therapy in maintaining short-term remission in children with INS dependent on both drugs and allow their temporary withdrawal.
Figures
References
-
- McEnery PT, Strife CF: Nephrotic syndrome in childhood. Management and treatment in patients with minimal change disease, mesangial proliferation, or focal glomerulosclerosis. Pediatr Clin North Am 29: 875–894, 1982 - PubMed
-
- Tryggvason K, Wartiovaara J: Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens 10: 543–549, 2001 - PubMed
-
- Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 - PubMed
-
- Caridi G, Bertelli R, Di Duca M, Dagnino M, Emma F, Onetti Muda A, Scolari F, Miglietti N, Mazzucco G, Murer L, Carrea A, Massella L, Rizzoni G, Perfumo F, Ghiggeri GM: Broadening the spectrum of diseases related to podocin mutations. J Am Soc Nephrol 14: 1278–1286, 2003 - PubMed
-
- Cohen AH, Border WA, Glassock RJ: Nehprotic syndrome with glomerular mesangial IgM deposits. Lab Invest 38: 610–619, 1978 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

