Quantitative mass spectrometry catalogues Salmonella pathogenicity island-2 effectors and identifies their cognate host binding partners
- PMID: 21566117
- PMCID: PMC3129184
- DOI: 10.1074/jbc.M111.224600
Quantitative mass spectrometry catalogues Salmonella pathogenicity island-2 effectors and identifies their cognate host binding partners
Abstract
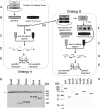
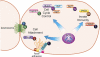
Gram-negative bacterial pathogens have developed specialized secretion systems to transfer bacterial proteins directly into host cells. These bacterial effectors are central to virulence and reprogram host cell processes to favor bacterial survival, colonization, and proliferation. Knowing the complete set of effectors encoded by a particular pathogen is the key to understanding bacterial disease. In addition, the identification of the molecular assemblies that these effectors engage once inside the host cell is critical to determining the mechanism of action of each effector. In this work we used stable isotope labeling of amino acids in cell culture (SILAC), a powerful quantitative proteomics technique, to identify the proteins secreted by the Salmonella pathogenicity island-2 type three secretion system (SPI-2 T3SS) and to characterize the host interaction partners of SPI-2 effectors. We confirmed many of the known SPI-2 effectors and were able to identify several novel substrate candidates of this secretion system. We verified previously published host protein-effector binding pairs and obtained 11 novel interactions, three of which were investigated further and confirmed by reciprocal co-immunoprecipitation. The host cell interaction partners identified here suggest that Salmonella SPI-2 effectors target, in a concerted fashion, cellular processes such as cell attachment and cell cycle control that are underappreciated in the context of infection. The technology outlined in this study is specific and sensitive and serves as a robust tool for the identification of effectors and their host targets that is readily amenable to the study of other bacterial pathogens.
Figures



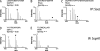

References
-
- Galán J. E., Wolf-Watz H. (2006) Nature 444, 567–573 - PubMed
-
- Valdez Y., Ferreira R. B., Finlay B. B. (2009) Curr. Top. Microbiol. Immunol. 337, 93–127 - PubMed
-
- Marcus S. L., Brumell J. H., Pfeifer C. G., Finlay B. B. (2000) Microbes Infect. 2, 145–156 - PubMed
-
- Menendez A., Arena E. T., Guttman J. A., Thorson L., Vallance B. A., Vogl W., Finlay B. B. (2009) J. Infect. Dis. 200, 1703–1713 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

