Molecular insight into how HIV-1 Vpr protein impairs cell growth through two genetically distinct pathways
- PMID: 21566118
- PMCID: PMC3129155
- DOI: 10.1074/jbc.M111.220780
Molecular insight into how HIV-1 Vpr protein impairs cell growth through two genetically distinct pathways
Abstract
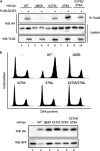
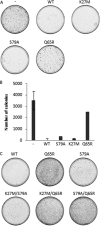
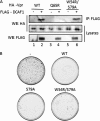
Vpr, a small HIV auxiliary protein, hijacks the CUL4 ubiquitin ligase through DCAF1 to inactivate an unknown cellular target, leading to cell cycle arrest at the G(2) phase and cell death. Here we first sought to delineate the Vpr determinants involved in the binding to DCAF1 and to the target. On the one hand, the three α-helices of Vpr are necessary and sufficient for binding to DCAF1; on the other hand, nonlinear determinants in Vpr are required for binding to the target, as shown by using protein chimeras. We also underscore that a SRIG motif conserved in the C-terminal tail of Vpr proteins from HIV-1/SIVcpz and HIV-2/SIVsmm lineages is critical for G(2) arrest. Our results suggest that this motif may be predictive of the ability of Vpr proteins from other SIV lineages to mediate G(2) arrest. We took advantage of the characterization of a subset of G(2) arrest-defective, but DCAF1 binding-proficient mutants, to investigate whether Vpr interferes with cell viability independently of its ability to induce G(2) arrest. These mutants inhibited cell colony formation in HeLa cells and are cytotoxic in lymphocytes, unmasking a G(2) arrest-independent cytopathic effect of Vpr. Furthermore these mutants do not block cell cycle progression at the G(1) or S phases but trigger apoptosis through caspase 3. Disruption of DCAF1 binding restored efficiency of colony formation. However, DCAF1 binding per se is not sufficient to confer cytopathicity. These data support a model in which Vpr recruits DCAF1 to induce the degradation of two host proteins independently required for proper cell growth.
Figures

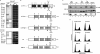


References
-
- Malim M. H., Emerman M. (2008) Cell Host Microbe 3, 388–398 - PubMed
-
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. (1987) Nature 328, 543–547 - PubMed
-
- Balliet J. W., Kolson D. L., Eiger G., Kim F. M., McGann K. A., Srinivasan A., Collman R. (1994) Virology 200, 623–631 - PubMed
-
- Connor R. I., Chen B. K., Choe S., Landau N. R. (1995) Virology 206, 935–944 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

