Transport mechanism and pH regulation of the Na+/H+ antiporter NhaA from Escherichia coli: an electrophysiological study
- PMID: 21566125
- PMCID: PMC3123120
- DOI: 10.1074/jbc.M111.230235
Transport mechanism and pH regulation of the Na+/H+ antiporter NhaA from Escherichia coli: an electrophysiological study
Abstract
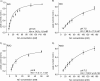
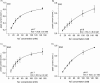
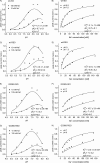
Using an electrophysiological assay the activity of NhaA was tested in a wide pH range from pH 5.0 to 9.5. Forward and reverse transport directions were investigated at zero membrane potential using preparations with inside-out and right side-out-oriented transporters with Na(+) or H(+) gradients as the driving force. Under symmetrical pH conditions with a Na(+) gradient for activation, both the wt and the pH-shifted G338S variant exhibit highly symmetrical transport activity with bell-shaped pH dependences, but the optimal pH was shifted 1.8 pH units to the acidic range in the variant. In both strains the pH dependence was associated with a systematic increase of the K(m) for Na(+) at acidic pH. Under symmetrical Na(+) concentration with a pH gradient for NhaA activation, an unexpected novel characteristic of the antiporter was revealed; rather than being down-regulated, it remained active even at pH as low as 5. These data allowed a transport mechanism to advance based on competing Na(+) and H(+) binding to a common transport site and a kinetic model to develop quantitatively explaining the experimental results. In support of these results, both alkaline pH and Na(+) induced the conformational change of NhaA associated with NhaA cation translocation as demonstrated here by trypsin digestion. Furthermore, Na(+) translocation was found to be associated with the displacement of a negative charge. In conclusion, the electrophysiological assay allows the revelation of the mechanism of NhaA antiport and sheds new light on the concept of NhaA pH regulation.
Figures






Similar articles
-
Differential effects of mutations on the transport properties of the Na+/H+ antiporter NhaA from Escherichia coli.J Biol Chem. 2013 Aug 23;288(34):24666-75. doi: 10.1074/jbc.M113.484071. Epub 2013 Jul 8. J Biol Chem. 2013. PMID: 23836890 Free PMC article.
-
A point mutation (G338S) and its suppressor mutations affect both the pH response of the NhaA-Na+/H+ antiporter as well as the growth phenotype of Escherichia coli.J Biol Chem. 1998 Oct 9;273(41):26470-6. doi: 10.1074/jbc.273.41.26470. J Biol Chem. 1998. PMID: 9756882
-
The monoclonal antibody 1F6 identifies a pH-dependent conformational change in the hydrophilic NH(2) terminus of NhaA Na(+)/H(+) antiporter of Escherichia coli.J Biol Chem. 2000 Feb 18;275(7):4734-42. doi: 10.1074/jbc.275.7.4734. J Biol Chem. 2000. PMID: 10671505
-
Prokaryotic Na+/H+ Exchangers-Transport Mechanism and Essential Residues.Int J Mol Sci. 2022 Aug 15;23(16):9156. doi: 10.3390/ijms23169156. Int J Mol Sci. 2022. PMID: 36012428 Free PMC article. Review.
-
The molecular mechanism of regulation of the NhaA Na+/H+ antiporter of Escherichia coli, a key transporter in the adaptation to Na+ and H+.Novartis Found Symp. 1999;221:183-96; discussion 196-9. doi: 10.1002/9780470515631.ch12. Novartis Found Symp. 1999. PMID: 10207920 Review.
Cited by
-
Differential effects of mutations on the transport properties of the Na+/H+ antiporter NhaA from Escherichia coli.J Biol Chem. 2013 Aug 23;288(34):24666-75. doi: 10.1074/jbc.M113.484071. Epub 2013 Jul 8. J Biol Chem. 2013. PMID: 23836890 Free PMC article.
-
Crystal structure of the Na+/H+ antiporter NhaA at active pH reveals the mechanistic basis for pH sensing.Nat Commun. 2022 Oct 26;13(1):6383. doi: 10.1038/s41467-022-34120-z. Nat Commun. 2022. PMID: 36289233 Free PMC article.
-
Mechanism of pH-dependent activation of the sodium-proton antiporter NhaA.Nat Commun. 2016 Oct 6;7:12940. doi: 10.1038/ncomms12940. Nat Commun. 2016. PMID: 27708266 Free PMC article.
-
General principles of secondary active transporter function.Biophys Rev (Melville). 2022 Mar;3(1):011307. doi: 10.1063/5.0047967. Epub 2022 Mar 29. Biophys Rev (Melville). 2022. PMID: 35434715 Free PMC article. Review.
-
pH- and sodium-induced changes in a sodium/proton antiporter.Elife. 2014 Jan 28;3:e01412. doi: 10.7554/eLife.01412. Elife. 2014. PMID: 24473071 Free PMC article.
References
-
- Brett C. L., Donowitz M., Rao R. (2005) Am. J. Physiol. Cell Physiol. 288, 223C–239C - PubMed
-
- Taglicht D., Padan E., Schuldiner S. (1991) J. Biol. Chem. 266, 11289–11294 - PubMed
-
- Padan E., Kozachkov L., Herz K., Rimon A. (2009) J. Exp. Biol. 212, 1593–1603 - PubMed
-
- Rothman A., Gerchman Y., Padan E., Schuldiner S. (1997) Biochemistry 36, 14572–14576 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

