Isopeptide ligation catalyzed by quintessential sortase A: mechanistic cues from cyclic and branched oligomers of indolicidin
- PMID: 21566128
- PMCID: PMC3129181
- DOI: 10.1074/jbc.M111.247650
Isopeptide ligation catalyzed by quintessential sortase A: mechanistic cues from cyclic and branched oligomers of indolicidin
Abstract
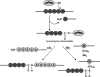
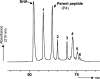
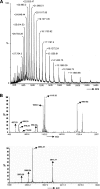
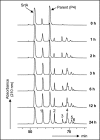
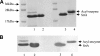
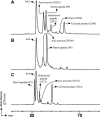
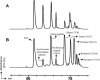
The housekeeping transpeptidase sortase A (SrtA) from Staphyloccocus aureus catalyzes the covalent anchoring of surface proteins to the cell wall by linking the threonyl carboxylate of the LPXTG recognition motif to the amino group of the pentaglycine cross-bridge of the peptidoglycan. SrtA-catalyzed ligation of an LPXTG containing polypeptide with an aminoglycine-terminated moiety occurs efficiently in vitro and has inspired the use of this enzyme as a synthetic tool in biological chemistry. Here we demonstrate the propensity of SrtA to catalyze "isopeptide" ligation. Using model peptide sequences, we show that SrtA can transfer LPXTG peptide substrates to the ε-amine of specific Lys residues and form cyclized and/or a gamut of branched oligomers. Our results provide insights about principles governing isopeptide ligation reactions catalyzed by SrtA and suggest that although cyclization is guided by distance relationship between Lys (ε-amine) and Thr (α-carboxyl) residues, facile branched oligomerization requires the presence of a stable and long-lived acyl-enzyme intermediate.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

