Comparative genomics of trace element dependence in biology
- PMID: 21566146
- PMCID: PMC3129141
- DOI: 10.1074/jbc.R110.172833
Comparative genomics of trace element dependence in biology
Abstract
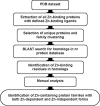
Biological trace elements are needed in small quantities but are used by all living organisms. A growing list of trace element-dependent proteins and trace element utilization pathways highlights the importance of these elements for life. In this minireview, we focus on recent advances in comparative genomics of trace elements and explore the evolutionary dynamics of the dependence of user proteins on these elements. Many zinc protein families evolved representatives that lack this metal, whereas selenocysteine in proteins is dynamically exchanged with cysteine. Several other elements, such as molybdenum and nickel, have a limited number of user protein families, but they are strictly dependent on these metals. Comparative genomics of trace elements provides a foundation for investigating the fundamental properties, functions, and evolutionary dynamics of trace element dependence in biology.
Figures

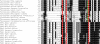

References
-
- Prentice A. M. (2005) Public Health Nutr. 8, 932–939 - PubMed
-
- Swinburn B. A., Ravussin E. (1994) Baillieres Clin. Endocrinol. Metab. 8, 527–548 - PubMed
-
- Fraga C. G. (2005) Mol. Aspects Med. 26, 235–244 - PubMed
-
- Baran E. J. (2004) Mini-Rev. Med. Chem. 4, 1–9 - PubMed
-
- Mertz W. (1998) Biol. Trace Elem. Res. 66, 185–191 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

