Two-photon absorption properties of proquinoidal D-A-D and A-D-A quadrupolar chromophores
- PMID: 21568299
- PMCID: PMC3121192
- DOI: 10.1021/jp2000738
Two-photon absorption properties of proquinoidal D-A-D and A-D-A quadrupolar chromophores
Abstract
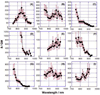
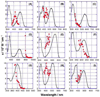
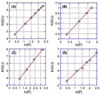
We report the synthesis, one- and two-photon absorption spectroscopy, fluorescence, and electrochemical properties of a series of quadrupolar molecules that feature proquinoidal π-aromatic acceptors. These quadrupolar molecules possess either donor-acceptor-donor (D-A-D) or acceptor-donor-acceptor (A-D-A) electronic motifs, and feature 4-N,N-dihexylaminophenyl, 4-dodecyloxyphenyl, 4-(N,N-dihexylamino)benzo[c][1,2,5]thiadiazolyl or 2,5-dioctyloxyphenyl electron donor moieties and benzo[c][1,2,5]thiadiazole (BTD) or 6,7-bis(3',7'-dimethyloctyl)[1,2,5]thiadiazolo[3,4-g]quinoxaline (TDQ) electron acceptor units. These conjugated structures are highly emissive in nonpolar solvents and exhibit large spectral red-shifts of their respective lowest energy absorption bands relative to analogous reference compounds that incorporate phenylene components in place of BTD and TDQ moieties. BTD-based D-A-D and A-D-A chromophores exhibit increasing fluorescence emission red-shifts, and a concomitant decrease of the fluorescence quantum yield (Φ(f)) with increasing solvent polarity; these data indicate that electronic excitation augments benzothiadiazole electron density via an internal charge transfer mechanism. The BTD- and TDQ-containing structures exhibit blue-shifted two-photon absorption (TPA) spectra relative to their corresponding one-photon absorption (OPA) spectra, and display high TPA cross sections (>100 GM) within these spectral windows. D-A-D and A-D-A structures that feature more extensive conjugation within this series of compounds exhibit larger TPA cross sections consistent with computational simulation. Factors governing TPA properties of these quadrupolar chromophores are discussed within the context of a three-state model.
Figures





Similar articles
-
Potentiometric, electronic structural, and ground- and excited-state optical properties of conjugated bis[(porphinato)zinc(II)] compounds featuring proquinoidal spacer units.J Am Chem Soc. 2005 Apr 13;127(14):5186-95. doi: 10.1021/ja040243h. J Am Chem Soc. 2005. PMID: 15810854
-
Novel 2,1,3-benzothiadiazole-based red-fluorescent dyes with enhanced two-photon absorption cross-sections.Chemistry. 2006 Mar 1;12(8):2303-17. doi: 10.1002/chem.200500921. Chemistry. 2006. PMID: 16363008
-
Donor-acceptor-donor thienyl/bithienyl-benzothiadiazole/quinoxaline model oligomers: experimental and theoretical studies.Phys Chem Chem Phys. 2013 Sep 28;15(36):15204-13. doi: 10.1039/c3cp52056k. Phys Chem Chem Phys. 2013. PMID: 23925140
-
Gigantic two-photon absorption cross sections and strong two-photon excited fluorescence in pyrene core dendrimers with fluorene/carbazole as dendrons and acetylene as linkages.J Phys Chem B. 2010 Sep 16;114(36):11737-45. doi: 10.1021/jp104868j. J Phys Chem B. 2010. PMID: 20735053
-
Large two-photon absorption of terpyridine-based quadrupolar derivatives: towards their applications in optical limiting and biological imaging.Chem Asian J. 2013 Mar;8(3):564-71. doi: 10.1002/asia.201201009. Epub 2012 Dec 28. Chem Asian J. 2013. PMID: 23281197
Cited by
-
Prospects for More Efficient Multi-Photon Absorption Photosensitizers Exhibiting Both Reactive Oxygen Species Generation and Luminescence.Molecules. 2021 Oct 19;26(20):6323. doi: 10.3390/molecules26206323. Molecules. 2021. PMID: 34684904 Free PMC article. Review.
-
A universal strategy for the fabrication of single-photon and multiphoton NIR nanoparticles by loading organic dyes into water-soluble polymer nanosponges.J Nanobiotechnology. 2022 Jul 6;20(1):311. doi: 10.1186/s12951-022-01515-5. J Nanobiotechnology. 2022. PMID: 35794602 Free PMC article.
-
Photoinduced Electron Transfer Across Phospholipid Bilayers in Anaerobic and Aerobic Atmospheres.Angew Chem Int Ed Engl. 2025 May 26;64(22):e202423393. doi: 10.1002/anie.202423393. Epub 2025 Apr 26. Angew Chem Int Ed Engl. 2025. PMID: 40095709 Free PMC article.
-
Laser-Induced Antibacterial Activity of Novel Symmetric Carbazole-Based Ethynylpyridine Photosensitizers.ACS Omega. 2018 Apr 30;3(4):3737-3743. doi: 10.1021/acsomega.8b00150. Epub 2018 Apr 3. ACS Omega. 2018. PMID: 30023877 Free PMC article.
-
Pyrrolo[3,2-b]pyrroles-From Unprecedented Solvatofluorochromism to Two-Photon Absorption.Chemistry. 2015 Dec 7;21(50):18364-74. doi: 10.1002/chem.201502762. Epub 2015 Oct 29. Chemistry. 2015. PMID: 26511232 Free PMC article.
References
-
- Parthenopoulos DA, Rentzepis PM. Science. 1989;245:843–845. - PubMed
-
- Strickler JH, Webb WW. Adv. Mater. 1993;5:479–481.
-
- Dvornikov AS, Walker EP, Rentzepis PM. J. Phys. Chem. A. 2009;113:13633–13644. - PubMed
-
- Zhou W, Kuebler SM, Braun KL, Yu T, Cammack JK, Ober CK, Perry JW, Marder SR. Science. 2002;296:1106–1109. - PubMed
-
- Pruzinsky SA, Braun PV. Adv. Funct. Mater. 2005;15:1995–2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

