A single chiroptical spectroscopic method may not be able to establish the absolute configurations of diastereomers: dimethylesters of hibiscus and garcinia acids
- PMID: 21568330
- PMCID: PMC3702036
- DOI: 10.1021/jp202501y
A single chiroptical spectroscopic method may not be able to establish the absolute configurations of diastereomers: dimethylesters of hibiscus and garcinia acids
Abstract
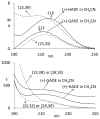
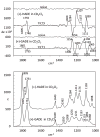
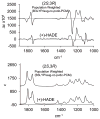
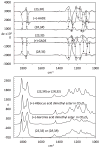
Electronic circular dichroism (ECD), optical rotatory dispersion (ORD), and vibrational circular dichroism (VCD) spectra of hibiscus acid dimethyl ester have been measured and analyzed in combination with quantum chemical calculations of corresponding spectra. These results, along with those reported previously for garcinia acid dimethyl ester, reveal that none of these three (ECD, ORD, or VCD) spectroscopic methods, in isolation, can unequivocally establish the absolute configurations of diastereomers. This deficiency is eliminated when a combined spectral analysis of either ECD and VCD or ORD and VCD methods is used. It is also found that the ambiguities in the assignment of absolute configurations of diastereomers may also be overcome when unpolarized vibrational absorption is included in the spectral analysis.
Figures


References
-
- Ibnusaud I, Thomas PT, Rani RN, Sasi PV, Beena T, Hisham A. Tetrahedron. 2002;58:4887–4892.
-
- Lewis YS, Neelakantan S, Anjanamurthy Curr Sci. 1964;3:82–83.
- Lewis YS, Neelakantan S. Phytochemistry. 1965;4:619–625.
-
- Jena BS, Jayaprakasha GK, Singh RP, Sakariah KK. J Agric Food Chem. 2002;50:10–22. - PubMed
-
- Boll PM, Sorensen E, Balieu E. Acta Chem Scand. 1969;23:286–293.
- Glusker JP, Minkin JA, Soule FB. Acta Crystallogr. 1972;B28:2499–2505.
-
- Autschbach J. Chirality. 2009;21:E116–E152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

