Carfilzomib: a novel second-generation proteasome inhibitor
- PMID: 21568676
- PMCID: PMC3931449
- DOI: 10.2217/fon.11.42
Carfilzomib: a novel second-generation proteasome inhibitor
Abstract
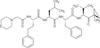
Carfilzomib (formerly PR-171) is a novel epoxyketone-based irreversible proteasome inhibitor. In preclinical studies, carfilzomib demonstrated irreversible binding to the proteasome and minimal off-target inhibition of other proteases. In clinical studies carfilzomib has demonstrated substantial antitumor activity in hematologic malignancies while exhibiting a well-tolerated side-effect profile. Painful neuropathy was minimally reported, suggesting a possible advantage over other proteasome inhibitors. With single-agent carfilzomib, dose-limiting toxicity was hematologic and included thrombocytopenia and neutropenia. In patients with relapsed or refractory multiple myeloma, twice-weekly consecutive-day single-agent carfilzomib 20 mg/m(2) for 3 weeks every 28 days, escalating to 27 mg/m(2) the second cycle was associated with a 54% overall response rate in bortezomib-naive patients and a 26% overall response rate in bortezomib and immunomodulatory drug refractory patients.
Conflict of interest statement
A Keith Stewart serves as a consultant for Onyx Pharmaceuticals, Millenium Pharmaceuticals, and receives honoraria from Celgene. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Figures
Similar articles
-
Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma.Blood. 2007 Nov 1;110(9):3281-90. doi: 10.1182/blood-2007-01-065888. Epub 2007 Jun 25. Blood. 2007. PMID: 17591945 Free PMC article.
-
An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib.Br J Haematol. 2012 Sep;158(6):739-48. doi: 10.1111/j.1365-2141.2012.09232.x. Epub 2012 Jul 30. Br J Haematol. 2012. PMID: 22845873 Free PMC article. Clinical Trial.
-
A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies.Clin Cancer Res. 2009 Nov 15;15(22):7085-91. doi: 10.1158/1078-0432.CCR-09-0822. Epub 2009 Nov 10. Clin Cancer Res. 2009. PMID: 19903785 Free PMC article. Clinical Trial.
-
Carfilzomib: a second-generation proteasome inhibitor for the treatment of relapsed and refractory multiple myeloma.Ann Pharmacother. 2013 Jan;47(1):56-62. doi: 10.1345/aph.1R561. Epub 2013 Jan 8. Ann Pharmacother. 2013. PMID: 23300152 Review.
-
Carfilzomib: a second-generation proteasome inhibitor for the treatment of multiple myeloma.Am J Health Syst Pharm. 2015 Mar 1;72(5):353-60. doi: 10.2146/ajhp130281. Am J Health Syst Pharm. 2015. PMID: 25694410 Review.
Cited by
-
Novel therapeutics in multiple myeloma.Hematology. 2012 Apr;17 Suppl 1(0 1):S105-8. doi: 10.1179/102453312X13336169156131. Hematology. 2012. PMID: 22507794 Free PMC article.
-
Emerging challenges in the design of selective substrates, inhibitors and activity-based probes for indistinguishable proteases.FEBS J. 2017 May;284(10):1518-1539. doi: 10.1111/febs.14001. Epub 2017 Jan 29. FEBS J. 2017. PMID: 28052575 Free PMC article. Review.
-
A Novel 3D High-Throughput Phenotypic Drug Screening Pipeline to Identify Drugs with Repurposing Potential for the Treatment of Ovarian Cancer.Adv Healthc Mater. 2025 Apr;14(11):e2404117. doi: 10.1002/adhm.202404117. Epub 2025 Mar 20. Adv Healthc Mater. 2025. PMID: 40109101 Free PMC article.
-
Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention.Neuro Oncol. 2012 Sep;14 Suppl 4(Suppl 4):iv45-54. doi: 10.1093/neuonc/nos203. Neuro Oncol. 2012. PMID: 23095830 Free PMC article. Review.
-
Posttranslational Regulation of Organic Anion Transporters by Ubiquitination: Known and Novel.Med Res Rev. 2016 Sep;36(5):964-79. doi: 10.1002/med.21397. Epub 2016 Jun 12. Med Res Rev. 2016. PMID: 27291023 Free PMC article. Review.
References
-
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl.J. Med. 2004;351:1860–1873. - PubMed
-
- Rajkumar SV, Kyle RA. Plasma Cell Disorders. In: Goldman L, Ausiello D, editors. Cecil Textbook of Medicine (23rd Edition) Philadelphia, PA, USA: Saunders; 2007. pp. 1426–1437.
-
- Ciechanover A. Proteolysis from the lysosome to ubiquitin and the proteasome. Nat. Rev. Mol. Cell Biol. 2005;6:79–87. - PubMed
-
- Dalton WS. The proteasome. Semin. Oncol. 2004;31:3–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials