Onset of efficacy and tolerability following the initiation dosing of long-acting paliperidone palmitate: post-hoc analyses of a randomized, double-blind clinical trial
- PMID: 21569242
- PMCID: PMC3115849
- DOI: 10.1186/1471-244X-11-79
Onset of efficacy and tolerability following the initiation dosing of long-acting paliperidone palmitate: post-hoc analyses of a randomized, double-blind clinical trial
Abstract
Background: Paliperidone palmitate is a long-acting injectable atypical antipsychotic for the acute and maintenance treatment of adults with schizophrenia. The recommended initiation dosing regimen is 234 mg on Day 1 and 156 mg on Day 8 via intramuscular (deltoid) injection; followed by 39 to 234 mg once-monthly thereafter (deltoid or gluteal). These post-hoc analyses addressed two commonly encountered clinical issues regarding the initiation dosing: the time to onset of efficacy and the associated tolerability.
Methods: In a 13-week double-blind trial, 652 subjects with schizophrenia were randomized to paliperidone palmitate 39, 156, or 234 mg (corresponding to 25, 100, or 150 mg equivalents of paliperidone, respectively) or placebo (NCT#00590577). Subjects randomized to paliperidone palmitate received 234 mg on Day 1, followed by their randomized fixed dose on Day 8, and monthly thereafter, with no oral antipsychotic supplementation. The onset of efficacy was defined as the first timepoint where the paliperidone palmitate group showed significant improvement in the Positive and Negative Syndrome Scale (PANSS) score compared to placebo (Analysis of Covariance [ANCOVA] models and Last Observation Carried Forward [LOCF] methodology without adjusting for multiplicity) using data from the Days 4, 8, 22, and 36 assessments. Adverse event (AE) rates and relative risks (RR) with 95% confidence intervals (CI) versus placebo were determined.
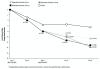
Results: Paliperidone palmitate 234 mg on Day 1 was associated with greater improvement than placebo on Least Squares (LS) mean PANSS total score at Day 8 (p=0.037). After the Day 8 injection of 156 mg, there was continued PANSS improvement at Day 22 (p≤0.007 vs. placebo) and Day 36 (p<0.001). Taken together with results in the 39 mg and 234 mg Day 8 arms, these findings suggest a trend towards a dose-dependent response. During Days 1 to 7, AEs reported in ≥2% of paliperidone palmitate subjects (234 mg) and a greater proportion of paliperidone palmitate than placebo subjects were: agitation (3.2% vs. 1.3%; RR 2.52 [95% CI 0.583, 10.904]), headache (4.0% vs. 3.8%; RR 1.06 [95% CI 0.433, 2.619]), and injection site pain (6.7% vs. 3.8%; RR 1.79 [95% CI 0.764, 4.208]). Days 8 to 36 AEs meeting the same criteria in the 156 mg Day 8 arm were: anxiety (3.1% vs. 2.5%; RR 1.24 [95% CI 0.340, 4.542]), psychotic disorder (2.5% vs. 1.3%; RR 1.99 [95% CI 0.369, 10.699]), dizziness (2.5% vs. 1.3%; RR 1.99 [95% CI 0.369, 10.699]), and injection site pain (2.5% vs. 1.3%; RR 1.99 [95% CI 0.369, 10.699]). Corresponding Days 8 to 36 AEs in the 39 mg Day 8 group were: agitation (4.5% vs. 4.4%; RR 1.03 [95% CI 0.371, 2.874]), anxiety (3.9% vs. 2.5%; RR 1.55 [95% CI 0.446, 5.381]), and psychotic disorder (2.6% vs. 1.3%; RR 2.07 [95% CI 0.384, 11.110]) while in the 234 mg Day 8 group it was anxiety (3.1% vs. 2.5%, RR 1.25 [95% CI 0.342, 4.570]).
Conclusions: Significantly greater symptom improvement was observed by Day 8 with paliperidone palmitate (234 mg on Day 1) compared to placebo; this effect was maintained after the 156 mg Day 8 injection, with a trend towards a dose-dependent response. No unexpected tolerability findings were noted in the first week or month after the initiation dosing.
Trial registration: ClinicalTrials.gov: NCT#00590577.
Trial registration: ClinicalTrials.gov NCT00590577.
Figures



References
-
- Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, Kreyenbuhl J. Am J Psychiatry. Second. 2 Suppl. Vol. 161. 2004. Practice guideline for the treatment of patients with schizophrenia; pp. 1–56. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

