Crystal structures of a halophilic archaeal malate synthase from Haloferax volcanii and comparisons with isoforms A and G
- PMID: 21569248
- PMCID: PMC3112382
- DOI: 10.1186/1472-6807-11-23
Crystal structures of a halophilic archaeal malate synthase from Haloferax volcanii and comparisons with isoforms A and G
Abstract
Background: Malate synthase, one of the two enzymes unique to the glyoxylate cycle, is found in all three domains of life, and is crucial to the utilization of two-carbon compounds for net biosynthetic pathways such as gluconeogenesis. In addition to the main isoforms A and G, so named because of their differential expression in E. coli grown on either acetate or glycolate respectively, a third distinct isoform has been identified. These three isoforms differ considerably in size and sequence conservation. The A isoform (MSA) comprises ~530 residues, the G isoform (MSG) is ~730 residues, and this third isoform (MSH-halophilic) is ~430 residues in length. Both isoforms A and G have been structurally characterized in detail, but no structures have been reported for the H isoform which has been found thus far only in members of the halophilic Archaea.
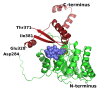
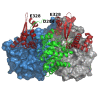
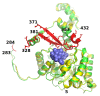
Results: We have solved the structure of a malate synthase H (MSH) isoform member from Haloferax volcanii in complex with glyoxylate at 2.51 Å resolution, and also as a ternary complex with acetyl-coenzyme A and pyruvate at 1.95 Å. Like the A and G isoforms, MSH is based on a β8/α8 (TIM) barrel. Unlike previously solved malate synthase structures which are all monomeric, this enzyme is found in the native state as a trimer/hexamer equilibrium. Compared to isoforms A and G, MSH displays deletion of an N-terminal domain and a smaller deletion at the C-terminus. The MSH active site is closely superimposable with those of MSA and MSG, with the ternary complex indicating a nucleophilic attack on pyruvate by the enolate intermediate of acetyl-coenzyme A.
Conclusions: The reported structures of MSH from Haloferax volcanii allow a detailed analysis and comparison with previously solved structures of isoforms A and G. These structural comparisons provide insight into evolutionary relationships among these isoforms, and also indicate that despite the size and sequence variation, and the truncated C-terminal domain of the H isoform, the catalytic mechanism is conserved. Sequence analysis in light of the structure indicates that additional members of isoform H likely exist in the databases but have been misannotated.
Figures
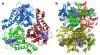








References
-
- McKinney JD, Honer zu Bentrup K, Munoz-Elias EJ, Miczak A, Chen B, Chan WT, Swenson D, Sacchettini JC, Jacobs WR Jr, Russell DG. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000;406(6797):735–738. doi: 10.1038/35021074. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

