Treatment effects of recombinant human soluble thrombomodulin in patients with severe sepsis: a historical control study
- PMID: 21569368
- PMCID: PMC3218981
- DOI: 10.1186/cc10228
Treatment effects of recombinant human soluble thrombomodulin in patients with severe sepsis: a historical control study
Abstract
Introduction: Cross-talk between the coagulation system and inflammatory reactions during sepsis causes organ damage followed by multiple organ dysfunction syndrome or even death. Therefore, anticoagulant therapies have been expected to be beneficial in the treatment of severe sepsis. Recombinant human soluble thrombomodulin (rhTM) binds to thrombin to inactivate coagulation, and the thrombin-rhTM complex activates protein C to produce activated protein C. The purpose of this study was to examine the efficacy of rhTM for treating patients with sepsis-induced disseminated intravascular coagulation (DIC).
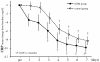
Methods: This study comprised 65 patients with sepsis-induced DIC who required ventilatory management. All patients fulfilled the criteria of severe sepsis and the International Society on Thrombosis and Haemostasis criteria for overt DIC. The initial 45 patients were treated without rhTM (control group), and the following 20 consecutive patients were treated with rhTM (0.06 mg/kg/day) for six days (rhTM group). The primary outcome measure was 28-day mortality. Stepwise multivariate Cox regression analysis was used to assess which independent variables were associated with mortality. Comparisons of Sequential Organ Failure Assessment (SOFA) score on sequential days between the two groups were analyzed by repeated measures analysis of variance.
Results: Cox regression analysis showed 28-day mortality to be significantly lower in the rhTM group than in the control group (adjusted hazard ratio, 0.303; 95% confidence interval, 0.106 to 0.871; P = 0.027). SOFA score in the rhTM group decreased significantly in comparison with that in the control group (P = 0.028). In the post hoc test, SOFA score decreased rapidly in the rhTM group compared with that in the control group on day 1 (P < 0.05).
Conclusions: We found that rhTM administration may improve organ dysfunction in patients with sepsis-induced DIC. Further clinical investigations are necessary to evaluate the effect of rhTM on the pathophysiology of sepsis-induced DIC.
Figures





References
-
- Ogura H, Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, Koseki K, Mayumi T, Murata A, Ikeda T, Ishikura H, Ueyama M, Kushimoto S, Saitoh D, Endo S, Shimazaki S. SIRS-associated coagulopathy and organ dysfunction in critically ill patients with thrombocytopenia. Shock. 2007;28:411–417. doi: 10.1097/shk.0b013e31804f7844. - DOI - PubMed
-
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

