Claudin-1 required for HCV virus entry has high potential for phosphorylation and O-glycosylation
- PMID: 21569618
- PMCID: PMC3115886
- DOI: 10.1186/1743-422X-8-229
Claudin-1 required for HCV virus entry has high potential for phosphorylation and O-glycosylation
Abstract
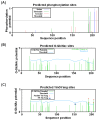
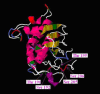
HCV is a leading cause of hepatocellular carcinoma and cirrhosis all over the world. Claudins belong to family of tight junction's proteins that are responsible for establishing barriers for controlling the flow of molecules around cells. For therapeutic strategies, regulation of viral entry into the host cells holds a lot of promise. During HCV infection claudin-1 is highly expressed in liver and believed to be associated with HCV virus entry after HCV binding with or without co-receptor CD81. The claudin-1 assembly with tight junctions is regulated by post translational modifications. During claudins assembly and disassembly with tight junctions, phosphorylation is required at C-terminal tail. In cellular proteins, interplay between phosphorylation and O-β-GlcNAc modification is believed to be functional switch, but it is very difficult to monitor these functional and vibrant changes in vivo. Netphos 2.0 and Disphos 1.3 programs were used for potential phosphorylation; NetPhosK 1.0 and KinasePhos for kinase prediction; and YinOYang 1.2 and OGPET to predict possible O-glycosylation sites. We also identified Yin Yang sites that may have potential for O-β-GlcNAc and phosphorylation interplay at same Ser/Thr residues. We for the first time proposed that alternate phosphorylation and O-β-GlcNAc modification on Ser 192, Ser 205, Ser 206; and Thr 191 may provide an on/off switch to regulate assembly of claudin-1 at tight junctions. In addition these phosphorylation sites may be targeted by novel chemotherapeutic agents to prevent phosphorylation lead by HCV viral entry complex.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

