Absence of the lysophosphatidic acid receptor LPA1 results in abnormal bone development and decreased bone mass
- PMID: 21569876
- PMCID: PMC3697734
- DOI: 10.1016/j.bone.2011.04.018
Absence of the lysophosphatidic acid receptor LPA1 results in abnormal bone development and decreased bone mass
Abstract
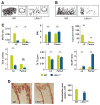
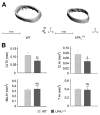
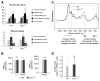
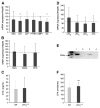
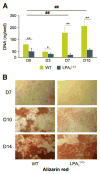
Lysophosphatidic acid (LPA) is a lipid mediator that acts in paracrine systems via interaction with a subset of G protein-coupled receptors (GPCRs). LPA promotes cell growth and differentiation, and has been shown to be implicated in a variety of developmental and pathophysiological processes. At least 6 LPA GPCRs have been identified to date: LPA1-LPA6. Several studies have suggested that local production of LPA by tissues and cells contributes to paracrine regulation, and a complex interplay between LPA and its receptors, LPA1 and LPA4, is believed to be involved in the regulation of bone cell activity. In particular, LPA1 may activate both osteoblasts and osteoclasts. However, its role has not as yet been examined with regard to the overall status of bone in vivo. We attempted to clarify this role by defining the bone phenotype of LPA1((-/-)) mice. These mice demonstrated significant bone defects and low bone mass, indicating that LPA1 plays an important role in osteogenesis. The LPA1((-/-)) mice also presented growth and sternal and costal abnormalities, which highlights the specific roles of LPA1 during bone development. Microcomputed tomography and histological analysis demonstrated osteoporosis in the trabecular and cortical bone of LPA1((-/-)) mice. Finally, bone marrow mesenchymal progenitors from these mice displayed decreased osteoblastic differentiation. These results suggest that LPA1 strongly influences bone development both qualitatively and quantitatively and that, in vivo, its absence results in decreased osteogenesis with no clear modification of osteoclasis. They open perspectives for a better understanding of the role of the LPA/LPA1 paracrine pathway in bone pathophysiology.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest. All authors have no conflict of interest.
Figures

References
-
- Ralston SH, Uitterlinden AG. Genetics of osteoporosis. Endocr Rev. 2010;31:629–62. - PubMed
-
- Fourcade O, Simon MF, Viode C, Rugani N, Leballe F, Ragab A, et al. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 1995;80:919–27. - PubMed
-
- Gaits F, Fourcade O, Le Balle F, Gueguen G, Gaige B, Gassama-Diagne A, et al. Lysophosphatidic acid as a phospholipid mediator: pathways of synthesis. FEBS Lett. 1997;410:54–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

