Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial
- PMID: 21570111
- PMCID: PMC3109515
- DOI: 10.1016/S0140-6736(11)60399-1
Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial
Abstract
Background: Elderly and frail patients with cancer, although often treated with chemotherapy, are under-represented in clinical trials. We designed FOCUS2 to investigate reduced-dose chemotherapy options and to seek objective predictors of outcome in frail patients with advanced colorectal cancer.
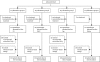
Methods: We undertook an open, 2 × 2 factorial trial in 61 UK centres for patients with previously untreated advanced colorectal cancer who were considered unfit for full-dose chemotherapy. After comprehensive health assessment (CHA), patients were randomly assigned by minimisation to: 48-h intravenous fluorouracil with levofolinate (group A); oxaliplatin and fluorouracil (group B); capecitabine (group C); or oxaliplatin and capecitabine (group D). Treatment allocation was not masked. Starting doses were 80% of standard doses, with discretionary escalation to full dose after 6 weeks. The two primary outcome measures were: addition of oxaliplatin ([A vs B] + [C vs D]), assessed with progression-free survival (PFS); and substitution of fluorouracil with capecitabine ([A vs C] + [B vs D]), assessed by change from baseline to 12 weeks in global quality of life (QoL). Analysis was by intention to treat. Baseline clinical and CHA data were modelled against outcomes with a novel composite measure, overall treatment utility (OTU). This study is registered, number ISRCTN21221452.
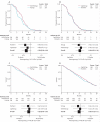
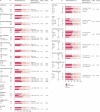
Findings: 459 patients were randomly assigned (115 to each of groups A-C, 114 to group D). Factorial comparison of addition of oxaliplatin versus no addition suggested some improvement in PFS, but the finding was not significant (median 5·8 months [IQR 3·3-7·5] vs 4·5 months [2·8-6·4]; hazard ratio 0·84, 95% CI 0·69-1·01, p=0·07). Replacement of fluorouracil with capecitabine did not improve global QoL: 69 of 124 (56%) patients receiving fluorouracil reported improvement in global QoL compared with 69 of 123 (56%) receiving capecitabine. The risk of having any grade 3 or worse toxic effect was not significantly increased with oxaliplatin (83/219 [38%] vs 70/221 [32%]; p=0·17), but was higher with capecitabine than with fluorouracil (88/222 [40%] vs 65/218 [30%]; p=0·03). In multivariable analysis, fewer baseline symptoms (odds ratio 1·32, 95% CI 1·14-1·52), less widespread disease (1·51, 1·05-2·19), and use of oxaliplatin (0·57, 0·39-0·82) were predictive of better OTU.
Interpretation: FOCUS2 shows that with an appropriate design, including reduced starting doses of chemotherapy, frail and elderly patients can participate in a randomised controlled trial. On balance, a combination including oxaliplatin was preferable to single-agent fluoropyrimidines, although the primary endpoint of PFS was not met. Capecitabine did not improve QoL compared with fluorouracil. Comprehensive baseline assessment holds promise as an objective predictor of treatment benefit.
Funding: Cancer Research UK and the Medical Research Council.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Do we need oncology trials tailored for the elderly or frail?Lancet. 2011 May 21;377(9779):1725-7. doi: 10.1016/S0140-6736(11)60654-5. Lancet. 2011. PMID: 21601694 No abstract available.
-
Chemotherapy for older patients with colorectal cancer.Lancet. 2011 Aug 27;378(9793):765; author reply 765-6. doi: 10.1016/S0140-6736(11)61368-8. Lancet. 2011. PMID: 21872740 No abstract available.
-
Chemotherapy for older patients with colorectal cancer.Lancet. 2011 Aug 27;378(9793):765; author reply 765-6. doi: 10.1016/S0140-6736(11)61367-6. Lancet. 2011. PMID: 21872741 No abstract available.
-
[Chemotherapy in elderly frail patients with advanced colorectal cancer. MRC FOCUS 2 (UK Medical Research Council Fluorouracil, Oxaliplatin, and Capecitabine: Use and Sequencing)].Internist (Berl). 2012 May;53(5):635-9. doi: 10.1007/s00108-012-3065-y. Internist (Berl). 2012. PMID: 22527668 German. No abstract available.
References
-
- Ferlay J, Autier P, Boniol M. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. - PubMed
-
- American Cancer Society . Cancer facts and figures, 2006. American Cancer Society; Atlanta: 2006. http://www.cancer.org/downloads/STT/CAFF2006PWSecured.pdf (accessed April 26, 2011).
-
- UK Office of National Statistics Mortality statistics. 2007 registrations. http://www.statistics.gov.uk/STATBASE/Expodata/Spreadsheets/D9784.csv (accessed April 26, 2011).
-
- Folprecht G, Seymour MT, Saltz L. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2691 patients in randomized controlled trials. J Clin Oncol. 2008;26:1443–1451. - PubMed
-
- De Gramont A, Figer A, Seymour MT. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

