MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between
- PMID: 21570275
- PMCID: PMC3148405
- DOI: 10.1016/j.ceb.2011.04.008
MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between
Abstract
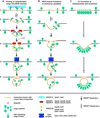
Multivesicular bodies (MVBs) are endosomes that have internalized portions of the limiting membrane into the compartment, thereby forming intralumenal vesicles. This vesicle formation is unusual in that it is directed away from the cytoplasm, which requires a unique mechanism unlike any mechanism described for other vesicle formation events. The best contenders for the machinery that drives MVB vesicle formation are the ESCRT protein complexes. However, increasing evidence suggests that lipids may play a key role in this membrane-deformation process. This review attempts to combine the seemingly contradictory findings into a MVB vesicle formation model that is based on a lipid-driven and ESCRT-regulated mechanism.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
References
-
- Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

