Decellularized tissue-engineered blood vessel as an arterial conduit
- PMID: 21571635
- PMCID: PMC3107282
- DOI: 10.1073/pnas.1019506108
Decellularized tissue-engineered blood vessel as an arterial conduit
Abstract
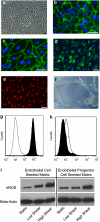
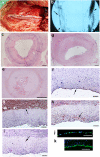
Arterial tissue-engineering techniques that have been reported previously typically involve long waiting times of several months while cells from the recipient are cultured to create the engineered vessel. In this study, we developed a different approach to arterial tissue engineering that can substantially reduce the waiting time for a graft. Tissue-engineered vessels (TEVs) were grown from banked porcine smooth muscle cells that were allogeneic to the intended recipient, using a biomimetic perfusion system. The engineered vessels were then decellularized, leaving behind the mechanically robust extracellular matrix of the graft wall. The acellular grafts were then seeded with cells that were derived from the intended recipient--either endothelial progenitor cells (EPC) or endothelial cell (EC)--on the graft lumen. TEV were then implanted as end-to-side grafts in the porcine carotid artery, which is a rigorous testbed due to its tendency for graft occlusion. The EPC- and EC-seeded TEV all remained patent for 30 d in this study, whereas the contralateral control vein grafts were patent in only 3/8 implants. Going along with the improved patency, the cell-seeded TEV demonstrated less neointimal hyperplasia and fewer proliferating cells than did the vein grafts. Proteins in the mammalian target of rapamycin signaling pathway tended to be decreased in TEV compared with vein grafts, implicating this pathway in the TEV's resistance to occlusion from intimal hyperplasia. These results indicate that a readily available, decellularized tissue-engineered vessel can be seeded with autologous endothelial progenitor cells to provide a biological vascular graft that resists both clotting and intimal hyperplasia. In addition, these results show that engineered connective tissues can be grown from banked cells, rendered acellular, and then used for tissue regeneration in vivo.
Conflict of interest statement
Conflict of interest statement: L.E.N. has a financial interest in Humacyte, Inc., a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
Figures


References
-
- Yeager RA, et al. Differential patency and limb salvage for polytetrafluoroethylene and autogenous saphenous vein in severe lower extremity ischemia. Surgery. 1982;91:99–103. - PubMed
-
- Veith FJ, Moss CM, Sprayregen S, Montefusco C. Preoperative saphenous venography in arterial reconstructive surgery of the lower extremity. Surgery. 1979;85:253–256. - PubMed
-
- Giannoukas AD, et al. Pre-bypass quality assessment of the long saphenous vein wall with ultrasound and histology. Eur J Vasc Endovasc Surg. 1997;14:37–40. - PubMed
-
- Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. - PubMed
-
- Kakisis JD, Liapis CD, Breuer C, Sumpio BE. Artificial blood vessel: The Holy Grail of peripheral vascular surgery. J Vasc Surg. 2005;41:349–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

