Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity
- PMID: 21572097
- PMCID: PMC3155087
- DOI: 10.1093/toxsci/kfr116
Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity
Abstract
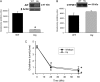
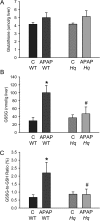
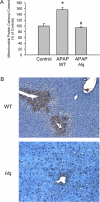
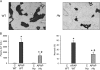
Acetaminophen (APAP) overdose causes liver injury in humans and mice. DNA fragmentation is a hallmark of APAP-induced cell death, and nuclear translocation of apoptosis-inducing factor (AIF) correlates with DNA fragmentation after APAP overdose. To test the hypothesis that AIF may be a critical mediator of APAP-induced cell death, fasted male AIF-deficient Harlequin (Hq) mice and respective wild-type (WT) animals were treated with 200 mg/kg APAP. At 6 h after APAP, WT animals developed severe liver injury as indicated by the increase in plasma alanine aminotransferase (ALT) activities (8600 ± 1870 U/l) and 61 ± 8% necrosis. This injury was accompanied by massive DNA strand breaks in centrilobular hepatocytes (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling [TUNEL] assay) and release of DNA fragments into the cytosol (anti-histone ELISA). In addition, there was formation of reactive oxygen (increase in liver glutathione disulfide (GSSG) levels and mitochondrial protein carbonyls) and peroxynitrite (nitrotyrosine [NT] staining) together with mitochondrial translocation of activated c-jun-N-terminal kinase (P-JNK) and release of AIF from the mitochondria. In contrast, Hq mice had significantly less liver injury (ALT: 330 ± 130 U/l; necrosis: 4 ± 2%), minimal nuclear DNA damage, and drastically reduced oxidant stress (based on all parameters) at 6 h. WT and Hq mice had the same baseline levels of cyp2E1 and of glutathione. The initial depletion of glutathione (20 min after APAP) was the same in both groups suggesting that there was no relevant difference in metabolic activation of APAP. Thus, AIF has a critical function in APAP hepatotoxicity by facilitating generation of reactive oxygen in mitochondria and, after nuclear translocation, AIF can be involved in DNA fragmentation.
Figures


References
-
- Apostolova N, Cervera AM, Victor VM, Cadenas S, Sanjuan-Pla A, Alvarez-Barrientos A, Esplugues JV, McCreath KJ. Loss of apoptosis-inducing factor leads to an increase in reactive oxygen species, and an impairment of respiration that can be reversed by antioxidants. Cell Death Differ. 2006;13:354–357. - PubMed
-
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol. Sci. 2006;94:217–225. - PubMed
-
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2008;324:8–14. - PubMed
-
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80:343–349. - PubMed
-
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol. Sci. 2000;58:109–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

