Mining a cathepsin inhibitor library for new antiparasitic drug leads
- PMID: 21572521
- PMCID: PMC3086806
- DOI: 10.1371/journal.pntd.0001023
Mining a cathepsin inhibitor library for new antiparasitic drug leads
Abstract
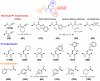
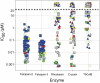
The targeting of parasite cysteine proteases with small molecules is emerging as a possible approach to treat tropical parasitic diseases such as sleeping sickness, Chagas' disease, and malaria. The homology of parasite cysteine proteases to the human cathepsins suggests that inhibitors originally developed for the latter may be a source of promising lead compounds for the former. We describe here the screening of a unique ∼ 2,100-member cathepsin inhibitor library against five parasite cysteine proteases thought to be relevant in tropical parasitic diseases. Compounds active against parasite enzymes were subsequently screened against cultured Plasmodium falciparum, Trypanosoma brucei brucei and/or Trypanosoma cruzi parasites and evaluated for cytotoxicity to mammalian cells. The end products of this effort include the identification of sub-micromolar cell-active leads as well as the elucidation of structure-activity trends that can guide further optimization efforts.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Rosenthal PJ. Antimalarial drug discovery: old and new approaches. Journal of Experimental Biology. 2003;206:3735–3744. - PubMed
-
- Renslo AR, McKerrow JH. Drug discovery and development for neglected parasitic diseases. Nat Chem Biol. 2006;2:701–710. - PubMed
-
- Bathurst I, Hentschel C. Medicines for Malaria Venture: sustaining antimalarial drug development. Trends Parasitol. 2006;22:301–307. - PubMed
-
- Castro JA, de Mecca MM, Bartel LC. Toxic side effects of drugs used to treat Chagas' disease (American trypanosomiasis). Hum Exp Toxicol. 2006;25:471–479. - PubMed

