3' processing of eukaryotic precursor tRNAs
- PMID: 21572561
- PMCID: PMC3092161
- DOI: 10.1002/wrna.64
3' processing of eukaryotic precursor tRNAs
Abstract
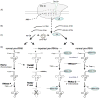
Biogenesis of eukaryotic tRNAs requires transcription by RNA polymerase III and subsequent processing. 5' processing of precursor tRNA occurs by a single mechanism, cleavage by RNase P, and usually occurs before 3' processing although some conditions allow observation of the 3'-first pathway. 3' processing is relatively complex and is the focus of this review. Precursor RNA 3'-end formation begins with pol III termination generating a variable length 3'-oligo(U) tract that represents an underappreciated and previously unreviewed determinant of processing. Evidence that the pol III-intrinsic 3'exonuclease activity mediated by Rpc11p affects 3'oligo(U) length is reviewed. In addition to multiple 3' nucleases, precursor tRNA(pre-tRNA) processing involves La and Lsm, distinct oligo(U)-binding proteins with proposed chaperone activities. 3' processing is performed by the endonuclease RNase Z or the exonuclease Rex1p (possibly others) along alternate pathways conditional on La. We review a Schizosaccharomyces pombe tRNA reporter system that has been used to distinguish two chaperone activities of La protein to its two conserved RNA binding motifs. Pre-tRNAs with structural impairments are degraded by a nuclear surveillance system that mediates polyadenylation by the TRAMP complex followed by 3'-digestion by the nuclear exosome which appears to compete with 3' processing. We also try to reconcile limited data on pre-tRNA processing and Lsm proteins which largely affect precursors but not mature tRNAs.A pathway is proposed in which 3' oligo(U) length is a primary determinant of La binding with subsequent steps distinguished by 3'-endo versus exo nucleases,chaperone activities, and nuclear surveillance.
Keywords: La protein; Lhp1; Lsm8; RNase P; Rex1; Rpc11; Rrp6; Sla1; TRAMP complex; Trf4; tRNase Z.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

