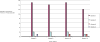Hepatotoxicity from first line antiretroviral therapy: an experience from a resource limited setting
- PMID: 21572852
- PMCID: PMC3092323
Hepatotoxicity from first line antiretroviral therapy: an experience from a resource limited setting
Abstract
Background: Highly active antiretroviral therapy (HAART) has been associated with liver toxicity. The role of monitoring for liver toxicity has not been well studied in resource-limited settings (RLS).
Objectives: To determine the background prevalence and incidence of liver injury and describe the associated signs and symptoms of acute hepatitis after initiating HAART; and to determine the role of liver enzyme tests in monitoring hepatotoxicity.
Methods: In this prospective study, in Mulago Hospital AIDS Clinics, we consecutively enrolled adult patients initiated on one of three first line HAART regimens [Stavudine (d4T)-Lamivudine (3TC) and nevirapine (NVP); Zidovudine (AZT)-3TC and Efavirenz (EFV) or d4T-3TC-EFV]. We monitored ALT (alanine aminotransferase) and clinical evidence of acute hepatitis at baseline, 2(nd), 6(th), 10(th) and 14(th) week of therapy.
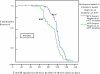
Results: Two hundred and forty HIV-positive HAART- naïve patients were enrolled in the study. The baseline prevalence of transaminitis was 1.7% with an incidence of 4.2% at 14 weeks. Grade 3-4 hepatotoxicity was documented in 1.3%. Jaundice was seen in grade 2-4 ALT elevations. Being on concurrent HAART and antituberculous drugs was associated with grade 2-4 toxicity compared to those who were only on HAART [OR; 16.0 (95% CI; 2.4-104.2)].
Conclusions: Incidence of severe hepatotoxicity within three months of first-line antiretroviral therapy was low, suggesting that routine measurement of transaminases may not be necessary in all patients initiating HAART in RLS. Routine measurement may be important in following patients on HAART and concurrent TB treatment as well as those with jaundice to avoid missing hepatotoxicity.
Keywords: HAART; Hepatotoxicity; Uganda.
Figures
References
-
- Laurent C, Diakhate N, Gueye NF, Toure MA, Sow PS, Faye MA, et al. The Senegalese government's highly active antiretroviral therapy initiative: an 18-month follow-up study. Aids. 2002;5; 16(10):1363–1370. - PubMed
-
- Weidle PJ, Mwebaze R, Sozi C, Rukundo G, Downing R, Hanson D, Ochola D, Mugyenyi P, Mermin J, et al. Assessment of a pilot antiretroviral drug therapy programme in Uganda: patients' response, survival, and drug resistance. Lancet Infect Dis. 2002;6(360(9326)):34–40. - PubMed
-
- Sanne I, Mommeja-Marin H, Hinkle J, Bartlett JA, Lederman MM, Maartens G, et al. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J Infect Dis. 2005;15;191(6):825–829. - PubMed
-
- Katabira ET, Kamya MR, editors. MOH. 2nd ed. Uganda: STD/AIDS Control Programme; 2008. National antiretroviral treatment and care guidelines for adults and children.
-
- Forna F LC, Solberg P, Asiimwe F, Were W, Mermin J, Behumbiize P, Tong T, et al. Clinical toxicity of highly active antiretroviral therapy in home-based-AIDS care program in rural Uganda. J acquir Imm Def Syndr. 2007;44:456–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical