Autonomic nervous dysfunction in hamsters infected with West Nile virus
- PMID: 21573009
- PMCID: PMC3090402
- DOI: 10.1371/journal.pone.0019575
Autonomic nervous dysfunction in hamsters infected with West Nile virus
Abstract
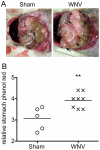
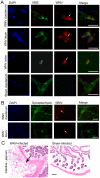
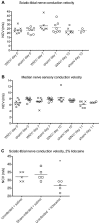
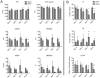
Clinical studies and case reports clearly document that West Nile virus (WNV) can cause respiratory and gastrointestinal (GI) complications. Other functions controlled by the autonomic nervous system may also be directly affected by WNV, such as bladder and cardiac functions. To investigate how WNV can cause autonomic dysfunctions, we focused on the cardiac and GI dysfunctions of rodents infected with WNV. Infected hamsters had distension of the stomach and intestines at day 9 after viral challenge. GI motility was detected by a dye retention assay; phenol red dye was retained more in the stomachs of infected hamsters as compared to sham-infected hamsters. The amplitudes of electromygraphs (EMGs) of intestinal muscles were significantly reduced. Myenteric neurons that innervate the intestines, in addition to neurons in the brain stem, were identified to be infected with WNV. These data suggest that infected neurons controlling autonomic function were the cause of GI dysfunction in WNV-infected hamsters. Using radiotelemetry to record electrocardiograms and to measure heart rate variability (HRV), a well-accepted readout for autonomic function, we determined that HRV and autonomic function were suppressed in WNV-infected hamsters. Cardiac histopathology was observed at day 9 only in the right atrium, which was coincident with WNV staining. A subset of WNV infected cells was identified among cells with hyperpolarization-activated cyclic nucleotide-gated potassium channel 4 (HCN4) as a marker for cells in the sinoatrial (SA) and atrioventricular (AV) nodes. The unique contribution of this study is the discovery that WNV infection of hamsters can lead to autonomic dysfunction as determined by reduced HRV and reduced EMG amplitudes of the GI tract. These data may model autonomic dysfunction of the human West Nile neurological disease.
Conflict of interest statement
Figures

Similar articles
-
Autonomic deficit not the cause of death in West Nile virus neurological disease.Clin Auton Res. 2014 Feb;24(1):15-23. doi: 10.1007/s10286-013-0213-y. Epub 2013 Oct 25. Clin Auton Res. 2014. PMID: 24158383 Free PMC article.
-
Neurological suppression of diaphragm electromyographs in hamsters infected with West Nile virus.J Neurovirol. 2010 Jul;16(4):318-29. doi: 10.3109/13550284.2010.501847. J Neurovirol. 2010. PMID: 20632796 Free PMC article.
-
Phrenic nerve deficits and neurological immunopathology associated with acute West Nile virus infection in mice and hamsters.J Neurovirol. 2017 Apr;23(2):186-204. doi: 10.1007/s13365-016-0488-6. Epub 2016 Oct 19. J Neurovirol. 2017. PMID: 27761801 Free PMC article.
-
Neurological approaches for investigating West Nile virus disease and its treatment in rodents.Antiviral Res. 2013 Nov;100(2):535-45. doi: 10.1016/j.antiviral.2013.09.010. Epub 2013 Sep 19. Antiviral Res. 2013. PMID: 24055448 Free PMC article. Review.
-
West Nile virus and kidney disease.Expert Rev Anti Infect Ther. 2013 May;11(5):479-87. doi: 10.1586/eri.13.34. Expert Rev Anti Infect Ther. 2013. PMID: 23627854 Review.
Cited by
-
Current Understanding of West Nile Virus Clinical Manifestations, Immune Responses, Neuroinvasion, and Immunotherapeutic Implications.Pathogens. 2019 Oct 16;8(4):193. doi: 10.3390/pathogens8040193. Pathogens. 2019. PMID: 31623175 Free PMC article. Review.
-
Autonomic deficit not the cause of death in West Nile virus neurological disease.Clin Auton Res. 2014 Feb;24(1):15-23. doi: 10.1007/s10286-013-0213-y. Epub 2013 Oct 25. Clin Auton Res. 2014. PMID: 24158383 Free PMC article.
-
Assessment of Autonomic Nervous System Function in Patients with Chronic Fatigue Syndrome and Post-COVID-19 Syndrome Presenting with Recurrent Syncope.J Clin Med. 2025 Jan 26;14(3):811. doi: 10.3390/jcm14030811. J Clin Med. 2025. PMID: 39941481 Free PMC article.
-
Intestinal Dysmotility Syndromes following Systemic Infection by Flaviviruses.Cell. 2018 Nov 15;175(5):1198-1212.e12. doi: 10.1016/j.cell.2018.08.069. Epub 2018 Oct 4. Cell. 2018. PMID: 30293866 Free PMC article.
-
Flavivirus Persistence in Wildlife Populations.Viruses. 2021 Oct 18;13(10):2099. doi: 10.3390/v13102099. Viruses. 2021. PMID: 34696529 Free PMC article. Review.
References
-
- Herlocher ML, Truscon R, Elias S, Yen HL, Roberts NA, et al. Influenza viruses resistant to the antiviral drug oseltamivir: transmission studies in ferrets. J Infect Dis. 2004;190:1627–1630. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

