Crystal structures of two aminoglycoside kinases bound with a eukaryotic protein kinase inhibitor
- PMID: 21573013
- PMCID: PMC3090406
- DOI: 10.1371/journal.pone.0019589
Crystal structures of two aminoglycoside kinases bound with a eukaryotic protein kinase inhibitor
Abstract
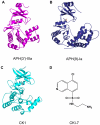
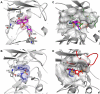
Antibiotic resistance is recognized as a growing healthcare problem. To address this issue, one strategy is to thwart the causal mechanism using an adjuvant in partner with the antibiotic. Aminoglycosides are a class of clinically important antibiotics used for the treatment of serious infections. Their usefulness has been compromised predominantly due to drug inactivation by aminoglycoside-modifying enzymes, such as aminoglycoside phosphotransferases or kinases. These kinases are structurally homologous to eukaryotic Ser/Thr and Tyr protein kinases and it has been shown that some can be inhibited by select protein kinase inhibitors. The aminoglycoside kinase, APH(3')-IIIa, can be inhibited by CKI-7, an ATP-competitive inhibitor for the casein kinase 1. We have determined that CKI-7 is also a moderate inhibitor for the atypical APH(9)-Ia. Here we present the crystal structures of CKI-7-bound APH(3')-IIIa and APH(9)-Ia, the first structures of a eukaryotic protein kinase inhibitor in complex with bacterial kinases. CKI-7 binds to the nucleotide-binding pocket of the enzymes and its binding alters the conformation of the nucleotide-binding loop, the segment homologous to the glycine-rich loop in eukaryotic protein kinases. Comparison of these structures with the CKI-7-bound casein kinase 1 reveals features in the binding pockets that are distinct in the bacterial kinases and could be exploited for the design of a bacterial kinase specific inhibitor. Our results provide evidence that an inhibitor for a subset of APHs can be developed in order to curtail resistance to aminoglycosides.
Conflict of interest statement
Figures
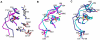
References
-
- Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;48:1–12. - PubMed
-
- De Pascale G, Wright GD. Antibiotic resistance by enzyme inactivation: from mechanisms to solutions. Chembiochem. 2010;11:1325–1334. - PubMed
-
- Therrien C, Levesque RC. Molecular basis of antibiotic resistance and beta-lactamase inhibition by mechanism-based inactivators: perspectives and future directions. FEMS Microbiol Rev. 2000;24:251–262. - PubMed
-
- Burk DL, Berghuis AM. Protein kinase inhibitors and antibiotic resistance. Pharmacol Ther. 2002;93:283–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

