Protein kinase B (Akt) and mitogen-activated protein kinase p38α in retinal ischemic post-conditioning
- PMID: 21573888
- PMCID: PMC5836301
- DOI: 10.1007/s12031-011-9523-5
Protein kinase B (Akt) and mitogen-activated protein kinase p38α in retinal ischemic post-conditioning
Abstract
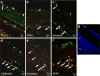
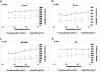
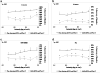
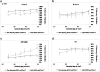
In previous studies, it was shown that post-conditioning, a transient period of brief ischemia following prolonged severe ischemia in the retina, could provide significant improvement in post-ischemic recovery, attenuation of cell loss, and decreased apoptosis. However, the mechanisms of post-conditioning in the retina have not been elucidated. We hypothesized that two kinases, mitogen-activated protein kinase p38α and protein kinase B (Akt), were involved in the mechanism of post-conditioning. Ischemia was induced in rat retina in vivo. Recovery after ischemia followed by 8 min of post-conditioning early in the reperfusion period after prolonged ischemia was assessed functionally (electroretinography) and histologically at 7 days after ischemia. We examined the role of p38α and Akt subtypes 1-3 in post-conditioning by intravitreal injection of interfering RNA 6 h prior to ischemia and post-conditioning and compared the results to injection of non-silencing interfering RNA sequence. The blockade of p38α significantly decreased the recovery after ischemia and post-conditioning, and enhanced cell loss and disorganization of the retina. Blockade of Akt1, and to a lesser degree, Akt2, significantly decreased the recovery after ischemia and enhanced cell loss and disorganization. These differences in the effects of blockade of Akt subtypes were not explainable by distribution of Akt subtypes in the retina, which were similar. In conclusion, both p38 and Akt are essential components of the neuroprotection induced by post-ischemic conditioning in the retina.
Conflict of interest statement
There is no conflict of interest or commercial interest for any of the authors.
Figures




References
-
- Bui BV, Edmunds B, Cioffi GA, Fortune B. The gradient of retinal functional changes during acute intraocular pressure elevation. Invest Ophthalmol Vis Sci. 2005;46:202–213. - PubMed
-
- Dorsett Y, Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev. 2004;3:318–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

