Smoking cessation in primary care - a randomized controlled trial of bupropione, nicotine replacements, CBT and a minimal intervention
- PMID: 21574208
- PMCID: PMC6878499
- DOI: 10.1002/mpr.328
Smoking cessation in primary care - a randomized controlled trial of bupropione, nicotine replacements, CBT and a minimal intervention
Abstract
Background/aims: Smoking cessation has been shown to be effective in randomized controlled trials. It is unclear though, whether interventions also work in routine primary care.
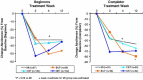
Methods: In 167 primary care settings we conducted a randomized four-armed smoking cessation trial to examine the efficacy of a minimal intervention (MI; n = 81), cognitive-behavioral therapy (CBT; n = 175), bupropion (BUP; n = 108) and nicotine replacements (NRT; n = 103). Overall, 467 current smokers were enrolled. Abstinence rates at the end of treatment (12 weeks) were 32.8% for MI patients, 34.8% for CBT, 35.3% for NRT, and 46.5% for BUP patients (ITT, intention to treat) (no differential effects). Retention rates were highest in the BUP group (59.3%) and lowest in the NRT group (50.5%). Completer findings were: MI, 56.4%; CBT, 64%; BUP, 79.3%; NRT, 69.2% (LOCF, lost to follow-up). No serious adverse events occurred during or after the medication phase. At 12-month follow-up continuous abstinence rates were: BUP, 29.0%; CBT, 20.9%; NRT, 29.6%; MI, 29.6%.
Conclusion: Our findings suggest that established smoking cessation treatments are effective when applied by non-specialist primary care physicians. Our data supports a structured, multimodal treatment structure as core ingredient of successful smoking cessation in primary care.
Copyright © 2011 John Wiley & Sons, Ltd.
Figures
References
-
- American Psychiatric Association (APA) (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Washington DC, APA.
-
- Cottler L.B. (1990) The CIDI and CIDI‐substance abuse module (SAM): cross‐cultural instruments for assessing DSM‐III, DSM‐III‐R and ICD‐10 criteria. NIDA Research Monograph, 105, 220–226. - PubMed
-
- Cottler L.B., Robins L.N., Grant B.F., Blaine J., Towle L.H., Wittchen H.U., Sartorius N. (1991) The CIDI‐Core Substance ‐abuse and dependence questions ‐ cross‐cultural and nosological issues. British Journal of Psychiatry, 159, 653–658. - PubMed
-
- DiClemente C.C., Prochaska J.O., Fairhurst S.K., Velicer W.F., Velasquez M.M., Rossi J.S. (1991) The process of smoking cessation: an analysis of precontemplation, contemplation and preparation stages of change. Journal of Consulting and Clinical Psychology, 59, 295–304. - PubMed
-
- Fagerström K.O., Schneider N.G., Lunell E. (1993) Effectiveness of nicotine patch and nicotine gum as individual versus combined treatment for tobacco withdrawal symptoms. Psychopharmacology, 111(3), 271–277. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical