Value of large scale expansion of tumor infiltrating lymphocytes in a compartmentalised gas-permeable bag: interests for adoptive immunotherapy
- PMID: 21575188
- PMCID: PMC3125220
- DOI: 10.1186/1479-5876-9-63
Value of large scale expansion of tumor infiltrating lymphocytes in a compartmentalised gas-permeable bag: interests for adoptive immunotherapy
Abstract
Background: Adoptive cell therapy (ACT) has emerged as an effective treatment for patients with metastatic melanoma. However, there are several logistical and safety concerns associated with large-scale ex vivo expansion of tumour-specific T lymphocytes for widespread availability of ACT for cancer patients. To address these problems we developed a specific compartmentalised bag allowing efficient expansion of tumour-specific T lymphocytes in an easy handling, closed system.
Methods: Starting from lymph nodes from eight melanoma patients, we performed a side-by-side comparison of Tumour-Infiltrating Lymphocytes (TIL) produced after expansion in the compartmentalised bag versus TIL produced using the standard process in plates. Proliferation yield, viability, phenotype and IFNγ secretion were comparatively studied.
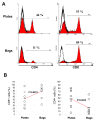
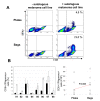
Results: We found no differences in proliferation yield and cell viability between both TIL production systems. Moreover, each of the cell products complied with our defined release criteria before being administered to the patient. The phenotype analysis indicated that the compartmentalised bag favours the expansion of CD8+ cells. Finally, we found that TIL stimulated in bags were enriched in reactive CD8+ T cells when co-cultured with the autologous melanoma cell line.
Conclusions: The stimulation of TIL with feeder cells in the specifically designed compartmentalised bag can advantageously replace the conventional protocol using plates. In particular, the higher expansion rate of reactive CD8+ T cells could have a significant impact for ACT.
Figures


Similar articles
-
TGF-beta1 induces preferential rapid expansion and persistence of tumor antigen-specific CD8+ T cells for adoptive immunotherapy.J Immunother. 2010 May;33(4):371-81. doi: 10.1097/CJI.0b013e3181cd1180. J Immunother. 2010. PMID: 20386469
-
Enrichment of CD8+ cells from melanoma tumor-infiltrating lymphocyte cultures reveals tumor reactivity for use in adoptive cell therapy.J Immunother. 2010 Jun;33(5):547-56. doi: 10.1097/CJI.0b013e3181d367bd. J Immunother. 2010. PMID: 20463593 Free PMC article.
-
High-scale expansion of melanoma-reactive TIL by a polyclonal stimulus: predictability and relation with disease advancement.Cancer Immunol Immunother. 2001 May;50(3):134-40. doi: 10.1007/PL00006683. Cancer Immunol Immunother. 2001. PMID: 11419180 Free PMC article.
-
Tumor-infiltrating lymphocytes in melanoma.Curr Oncol Rep. 2012 Oct;14(5):468-74. doi: 10.1007/s11912-012-0257-5. Curr Oncol Rep. 2012. PMID: 22878966 Free PMC article. Review.
-
White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: a report of the CTEP subcommittee on adoptive cell therapy.Clin Cancer Res. 2011 Apr 1;17(7):1664-73. doi: 10.1158/1078-0432.CCR-10-2272. Epub 2011 Feb 15. Clin Cancer Res. 2011. PMID: 21325070 Review.
Cited by
-
Ex vivo expansion of tumor-infiltrating lymphocytes from nasopharyngeal carcinoma patients for adoptive immunotherapy.Chin J Cancer. 2012 Jun;31(6):287-94. doi: 10.5732/cjc.011.10376. Epub 2012 Jan 17. Chin J Cancer. 2012. PMID: 22257383 Free PMC article.
-
Industrializing Autologous Adoptive Immunotherapies: Manufacturing Advances and Challenges.Front Med (Lausanne). 2018 May 23;5:150. doi: 10.3389/fmed.2018.00150. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29876351 Free PMC article. Review.
-
Enhanced metabolic activities for ATP production and elevated metabolic flux via pentose phosphate pathway contribute for better CIK cells expansion.Cell Prolif. 2019 May;52(3):e12594. doi: 10.1111/cpr.12594. Epub 2019 Mar 7. Cell Prolif. 2019. PMID: 30847992 Free PMC article.
-
Recent Advances in the Development of Bioreactors for Manufacturing of Adoptive Cell Immunotherapies.Bioengineering (Basel). 2022 Dec 15;9(12):808. doi: 10.3390/bioengineering9120808. Bioengineering (Basel). 2022. PMID: 36551014 Free PMC article. Review.
-
Summit on cell therapy for cancer: The importance of the interaction of multiple disciplines to advance clinical therapy.J Transl Med. 2011 Jul 8;9:107. doi: 10.1186/1479-5876-9-107. J Transl Med. 2011. PMID: 21740581 Free PMC article. Review.
References
-
- Marijt WA, Heemskerk MH, Kloosterboer FM, Goulmy E, Kester MG, van der Hoorn MA. et al.Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc Natl Acad Sci. 2003;100:2742–2747. doi: 10.1073/pnas.0530192100. - DOI - PMC - PubMed
-
- Figlin RA, Thompson JA, Bukowski RM, Vogelzang NJ, Novick AC, Lange P, Steinberg GD, Belldegrun AS. Multicenter, randomized, phase III trial of CD8(+) tumor-infiltrating lymphocytes in combination with recombinant interleukin-2 in metastatic renal cell carcinoma. J Clin Oncol. 1999;17:2521–2529. - PubMed
-
- Dreno B, Nguyen JM, Khammari A, Pandolfino MC, Tessier MH, Bercegeay S, Cassidanius A, Lemarre P, Billaudel S, Labarriere N, Jotereau F. Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother. 2002;51:539–546. doi: 10.1007/s00262-002-0315-1. - DOI - PMC - PubMed
-
- Khammari A, Nguyen JM, Pandolfino MC, Quereux G, Brocard A, Bercegeay S, Cassidanius A, Lemarre P, Volteau C, Labarriere N. et al.Long-term follow-up of patients treated by adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother. 2007;56:1853–1860. doi: 10.1007/s00262-007-0340-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

