Drosophila katanin-60 depolymerizes and severs at microtubule defects
- PMID: 21575578
- PMCID: PMC3093562
- DOI: 10.1016/j.bpj.2011.03.062
Drosophila katanin-60 depolymerizes and severs at microtubule defects
Abstract
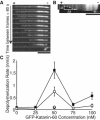
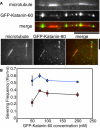
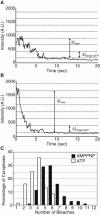
Microtubule (MT) length and location is tightly controlled in cells. One novel family of MT-associated proteins that regulates MT dynamics is the MT-severing enzymes. In this work, we investigate how katanin (p60), believed to be the first discovered severing enzyme, binds and severs MTs via single molecule total internal reflection fluorescence microscopy. We find that severing activity depends on katanin concentration. We also find that katanin can remove tubulin dimers from the ends of MTs, appearing to depolymerize MTs. Strikingly, katanin localizes and severs at the interface of GMPCPP-tubulin and GDP-tubulin suggesting that it targets to protofilament-shift defects. Finally, we observe that binding duration, mobility, and oligomerization are ATP dependent.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Conde C., Cáceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 2009;10:319–332. - PubMed
-
- Desai A., Mitchison T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997;13:83–117. - PubMed
-
- Hartman J.J., Mahr J., McNally F.J. Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell. 1998;93:277–287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

