Kinesin walks the line: single motors observed by atomic force microscopy
- PMID: 21575579
- PMCID: PMC3093571
- DOI: 10.1016/j.bpj.2011.04.015
Kinesin walks the line: single motors observed by atomic force microscopy
Abstract
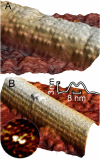
Motor proteins of the kinesin family move actively along microtubules to transport cargo within cells. How exactly a single motor proceeds on the 13 narrow lanes or protofilaments of a microtubule has not been visualized directly, and there persists controversy on the relative position of the two kinesin heads in different nucleotide states. We have succeeded in imaging Kinesin-1 dimers immobilized on microtubules with single-head resolution by atomic force microscopy. Moreover, we could catch glimpses of single Kinesin-1 dimers in their motion along microtubules with nanometer resolution. We find in our experiments that frequently both heads of one dimer are microtubule-bound at submicromolar ATP concentrations. Furthermore, we could unambiguously resolve that both heads bind to the same protofilament, instead of straddling two, and remain on this track during processive movement.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


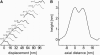
References
-
- Alberts B., Johnson A., Walter P. Garland Science; New York: 2008. Molecular Biology of the Cell.
-
- Vale R.D., Milligan R.A. The way things move: looking under the hood of molecular motor proteins. Science. 2000;288:88–95. - PubMed
-
- Veigel C., Schmidt C.F. Moving into the cell: single-molecule studies of molecular motors in complex environments. Nat. Rev. Mol. Cell Biol. 2011;12:163–176. - PubMed
-
- Kuo S.C., Gelles J., Sheetz M.P. A model for kinesin movement from nanometer-level movements of kinesin and cytoplasmic dynein and force measurements. J. Cell Sci. Suppl. 1991;14:135–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

