IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection
- PMID: 21575908
- PMCID: PMC3113467
- DOI: 10.1016/j.chom.2011.04.008
IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection
Abstract
Inflammasomes are cytoplasmic sensors of foreign molecules, including pathogens, and function to induce caspase-1 activation and IL-1β cytokine maturation. Whether such a mechanism exists in the nucleus and is effective against nuclear replicating pathogens is unknown. Nuclear replicating herpesvirus KSHV is associated with Kaposi Sarcoma, an angioproliferative tumor characterized by an inflammatory microenvironment including IL-1β. We demonstrate that during KSHV infection of endothelial cells, interferon gamma-inducible protein 16 (IFI16) interacts with the adaptor molecule ASC and procaspase-1 to form a functional inflammasome. This complex was initially detected in the nucleus and subsequently in the perinuclear area. KSHV gene expression and/or latent KSHV genome is required for inflammasome activation and IFI16 colocalizes with the KSHV genome in the infected cell nucleus. Caspase-1 activation by KSHV was reduced by IFI16 and ASC silencing. Our studies reveal IFI16 as a nuclear pathogen sensor and demonstrate that the inflammasome also functions in the nucleus.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
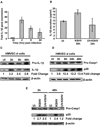

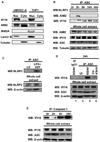



Comment in
-
Innate DNA sensing moves to the nucleus.Cell Host Microbe. 2011 May 19;9(5):351-3. doi: 10.1016/j.chom.2011.05.001. Cell Host Microbe. 2011. PMID: 21575905
References
-
- Aglipay JA, Lee SW, Okada S, Fujiuchi N, Ohtsuka T, Kwak JC, Wang Y, Johnstone RW, Deng C, Qin J, et al. A member of the Pyrin family, IFI16, is a novel BRCA1-associated protein involved in the p53-mediated apoptosis pathway. Oncogene. 2003;22:8931–8938. - PubMed
-
- Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

