Molecular characterization of a membrane-bound prenyltransferase specific for isoflavone from Sophora flavescens
- PMID: 21576242
- PMCID: PMC3129193
- DOI: 10.1074/jbc.M111.244426
Molecular characterization of a membrane-bound prenyltransferase specific for isoflavone from Sophora flavescens
Abstract
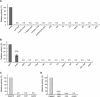
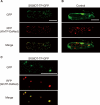
Prenylated isoflavones are secondary metabolites that are mainly distributed in legume plants. They often possess divergent biological activities such as anti-bacterial, anti-fungal, and anti-oxidant activities and thus attract much attention in food, medicinal, and agricultural research fields. Prenyltransferase is the key enzyme in the biosynthesis of prenylated flavonoids by catalyzing a rate-limiting step, i.e. the coupling process of two major metabolic pathways, the isoprenoid pathway and shikimate/polyketide pathway. However, so far only two genes have been isolated as prenyltransferases involved in the biosynthesis of prenylated flavonoids, namely naringenin 8-dimethylallyltransferase from Sophora flavescens (SfN8DT-1) specific for some limited flavanones and glycinol 4-dimethylallyltransferase from Glycine max (G4DT), specific for pterocarpan substrate. We have in this study isolated two novel genes coding for membrane-bound flavonoid prenyltransferases from S. flavescens, an isoflavone-specific prenyltransferase (SfG6DT) responsible for the prenylation of the genistein at the 6-position and a chalcone-specific prenyltransferase designated as isoliquiritigenin dimethylallyltransferase (SfiLDT). These prenyltransferases were enzymatically characterized using a yeast expression system. Analysis on the substrate specificity of chimeric enzymes between SfN8DT-1 and SfG6DT suggested that the determinant region for the specificity of the flavonoids was the domain neighboring the fifth transmembrane α-helix of the prenyltransferases.
Figures



References
-
- Tahara S., Ibrahim R. K. (1995) Phytochemistry 38, 1073–1094
-
- Barron D., Ibrahim R. K. (1996) Phytochemistry 43, 921–982
-
- Ahmed-Belkacem A., Pozza A., Muñoz-Martínez F., Bates S. E., Castanys S., Gamarro F., Di Pietro A., Pérez-Victoria J. M. (2005) Cancer Res. 65, 4852–4860 - PubMed
-
- Han A. R., Kang Y. J., Windono T., Lee S. K., Seo E. K. (2006) J. Nat. Prod. 69, 719–721 - PubMed
-
- Wang B. H., Ternai B., Polya G. (1997) Phytochemistry 44, 787–796 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

