Left ventricular systolic dysfunction induced by ventricular ectopy: a novel model for premature ventricular contraction-induced cardiomyopathy
- PMID: 21576277
- PMCID: PMC3175603
- DOI: 10.1161/CIRCEP.111.962381
Left ventricular systolic dysfunction induced by ventricular ectopy: a novel model for premature ventricular contraction-induced cardiomyopathy
Abstract
Background: Premature ventricular contractions (PVCs) commonly coexist with cardiomyopathy. Recently, PVCs have been identified as a possible cause of cardiomyopathy. We developed a PVC-induced cardiomyopathy animal model using a novel premature pacing algorithm to assess timeframe and reversibility of this cardiomyopathy and examine the associated histopathologic abnormalities.
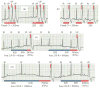
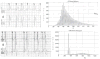
Methods and results: Thirteen mongrel dogs were implanted with a specially programmed pacemaker capable of simulating ventricular extrasystoles. Animals were randomly assigned to either 12 weeks of bigeminal PVCs (n = 7) or no PVCs (control, n = 6). Continuous 24-hour Holter monitoring corroborated ventricular bigeminy in the PVC group (PVC, 49.8% versus control, < 0.01%; P<0.0001). After 12 weeks, only the PVC group had cardiomyopathy, with a significant reduction in left ventricular ejection fraction (PVC, 39.7 ± 5.4% versus control, 60.7 ± 3.8%; P < 0.0001) and an increase in left ventricular end-systolic dimension (PVC, 33.3 ± 3.5 mm versus control, 23.7 ± 3.6 mm; P < 0.001). Ventricular effective refractory period showed a trend to prolong in the PVC group. PVC-induced cardiomyopathy was resolved within 2 to 4 weeks after discontinuation of PVCs. No inflammation, fibrosis, or changes in apoptosis and mitochondrial oxidative phosphorylation were observed with PVC-induced cardiomyopathy.
Conclusions: This novel PVC animal model demonstrates that frequent PVCs alone can induce a reversible form of cardiomyopathy in otherwise structurally normal hearts. PVC-induced cardiomyopathy lacks gross histopathologic and mitochondrial abnormalities seen in other canine models of cardiomyopathy.
Conflict of interest statement
Figures


References
-
- Bogun F, Crawford T, Reich S, Koelling TM, Armstrong W, Good E, Jongnarangsin K, Marine JE, Chugh A, Pelosi F, Oral H, Morady F. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: Comparison with a control group without intervention. Heart Rhythm. 2007;4:863–867. - PubMed
-
- Takemoto M, Yoshimura H, Ohba Y, Matsumoto Y, Yamamoto U, Mohri M, Yamamoto H, Origuchi H. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. Journal of the American College of Cardiology. 2005;45:1259–1265. - PubMed
-
- Niwano S, Wakisaka Y, Niwano H, Fukaya H, Kurokawa S, Kiryu M, Hatakeyama Y, Izumi T. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart (British Cardiac Society) 2009;95:1230–1237. - PubMed
-
- Yarlagadda RK, Iwai S, Stein KM, Markowitz SM, Shah BK, Cheung JW, Tan V, Lerman BB, Mittal S. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation. 2005;112:1092–1097. - PubMed
-
- Singh SN, Fletcher RD, Fisher SG, Singh BN, Lewis HD, Deedwania PC, Massie BM, Colling C, Lazzeri D. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival trial of antiarrhythmic therapy in congestive heart failure. The New England journal of medicine. 1995;333:77–82. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

