Innate immune responses to systemic Acinetobacter baumannii infection in mice: neutrophils, but not interleukin-17, mediate host resistance
- PMID: 21576323
- PMCID: PMC3147579
- DOI: 10.1128/IAI.00069-11
Innate immune responses to systemic Acinetobacter baumannii infection in mice: neutrophils, but not interleukin-17, mediate host resistance
Abstract
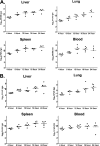
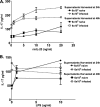
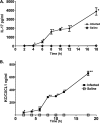
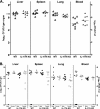
Acinetobacter baumannii is a nosocomial pathogen with a high prevalence of multiple-drug-resistant strains, causing pneumonia and sepsis. The current studies further develop a systemic mouse model of this infection and characterize selected innate immune responses to the organism. Five clinical isolates, with various degrees of antibiotic resistance, were assessed for virulence in two mouse strains, and between male and female mice, using intraperitoneal infection. A nearly 1,000-fold difference in virulence was found between bacterial strains, but no significant differences between sexes or mouse strains were observed. It was found that microbes disseminated rapidly from the peritoneal cavity to the lung and spleen, where they replicated. A persistent septic state was observed. The infection progressed rapidly, with mortality between 36 and 48 h. Depletion of neutrophils with antibody to Ly-6G decreased mean time to death and increased mortality. Interleukin-17 (IL-17) promotes the response of neutrophils by inducing production of the chemokine keratinocyte-derived chemoattractant (KC/CXCL1), the mouse homolog of human IL-8. Acinetobacter infection resulted in biphasic increases in both IL-17 and KC/CXCL1. Depletion of neither IL-17 nor KC/CXCL1, using specific antibodies, resulted in a difference in bacterial burdens in organs of infected mice at 10 h postinfection. Comparison of bacterial burdens between IL-17a(-/-) and wild-type mice confirmed that the absence of this cytokine did not sensitize mice to Acinetobacter infection. These studies definitely demonstrate the importance of neutrophils in resistance to systemic Acinetobacter infection. However, neither IL-17 nor KC/CXCL1 alone is required for effective host defense to systemic infection with this organism.
Figures



References
-
- Bernabeu-Wittel M., et al. 2005. Pharmacokinetic/pharmacodynamic assessment of the in-vivo efficacy of imipenem alone or in combination with amikacin for the treatment of experimental multiresistant Acinetobacter baumannii pneumonia. Clin. Microbiol. Infect. 11:319–325 - PubMed
-
- Brown C. R., Blaho V. A., Loiacono C. M. 2003. Susceptibility to experimental Lyme arthritis correlates with KC and monocyte chemoattractant protein-1 production in joints and requires neutrophil recruitment via CXCR2. J. Immunol. 171:893–901 - PubMed
-
- Brown H. J., et al. 2007. Toll-like receptor 4 ligation on intrinsic renal cells contributes to the induction of antibody-mediated glomerulonephritis via CXCL1 and CXCL2. J. Am. Soc. Nephrol. 18:1732–1739 - PubMed
-
- Centers for Disease Control Prevention. 2004. Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002-2004. MMWR Morb. Mortal. Wkly. Rep. 53:1063–1066 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

