Haemophilus ducreyi lipooligosaccharides induce expression of the immunosuppressive enzyme indoleamine 2,3-dioxygenase via type I interferons and tumor necrosis factor alpha in human dendritic cells
- PMID: 21576329
- PMCID: PMC3147552
- DOI: 10.1128/IAI.05021-11
Haemophilus ducreyi lipooligosaccharides induce expression of the immunosuppressive enzyme indoleamine 2,3-dioxygenase via type I interferons and tumor necrosis factor alpha in human dendritic cells
Abstract
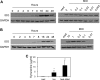
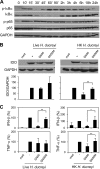
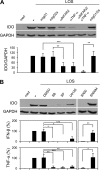
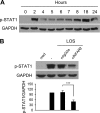
Haemophilus ducreyi causes chancroid, a genital ulcer disease. In human inoculation experiments, most volunteers fail to clear the bacteria despite the infiltration of innate and adaptive immune cells to the infected sites. The immunosuppressive protein indoleamine 2,3-dioxygenase (IDO) is a rate-limiting enzyme in the L-tryptophan-kynurenine metabolic pathway. Tryptophan depletion and tryptophan metabolites contribute to pathogen persistence by inhibiting T cell proliferation, inducing T cell apoptosis, and promoting the expansion of FOXP3(+) regulatory T (Treg) cells. We previously found that FOXP3(+) Treg cells are enriched in experimental lesions and that H. ducreyi induced IDO transcription in dendritic cells (DC) derived from blood of infected volunteers who developed pustules. Here, we showed that enzymatically active IDO was induced in DC by H. ducreyi. Neutralizing antibodies against interferon alpha/beta receptor 2 chain (IFNAR2) and tumor necrosis factor alpha (TNF-α) inhibited IDO induction. Inhibitors of the mitogen-activated protein kinase (MAPK) p38 and nuclear factor-κB (NF-κB) also inhibited IDO expression. Neither bacterial contact with nor uptake by DC was required for IDO activation. H. ducreyi culture supernatant and H. ducreyi lipooligosaccharides (LOS) induced IDO expression, which required type I interferons, TNF-α, and the three MAPK (p38, c-Jun N-terminal kinase, and extracellular signal regulated kinase) and NF-κB pathways. In addition, LOS-induced IFN-β activated the JAK-STAT pathway. Blocking the LOS/Toll-like receptor 4 (TLR4) signaling pathway greatly reduced H. ducreyi-induced IDO production. These findings indicate that H. ducreyi-induced IDO expression in DC is largely mediated by LOS via type I interferon- and TNF-α-dependent mechanisms and the MAPK, NF-κB, and JAK-STAT pathways.
Figures




Similar articles
-
A high-resolution view of the immune and stromal cell response to Haemophilus ducreyi infection in human volunteers.mBio. 2025 Mar 12;16(3):e0388524. doi: 10.1128/mbio.03885-24. Epub 2025 Jan 30. mBio. 2025. PMID: 39882906 Free PMC article.
-
Epstein-Barr virus infection induces indoleamine 2,3-dioxygenase expression in human monocyte-derived macrophages through p38/mitogen-activated protein kinase and NF-κB pathways: impairment in T cell functions.J Virol. 2014 Jun;88(12):6660-71. doi: 10.1128/JVI.03678-13. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696473 Free PMC article.
-
The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines.J Biochem. 2006 Apr;139(4):655-62. doi: 10.1093/jb/mvj072. J Biochem. 2006. PMID: 16672265
-
IDO-expressing regulatory dendritic cells in cancer and chronic infection.J Mol Med (Berl). 2008 Feb;86(2):145-60. doi: 10.1007/s00109-007-0262-6. Epub 2007 Sep 18. J Mol Med (Berl). 2008. PMID: 17876564 Review.
-
IDO/Kynurenine; novel insight for treatment of inflammatory diseases.Cytokine. 2023 Jun;166:156206. doi: 10.1016/j.cyto.2023.156206. Epub 2023 Apr 28. Cytokine. 2023. PMID: 37120946 Review.
Cited by
-
Indoleamine 2,3-dioxygenase expression regulates the survival and proliferation of Fusobacterium nucleatum in THP-1-derived macrophages.Cell Death Dis. 2018 Mar 2;9(3):355. doi: 10.1038/s41419-018-0389-0. Cell Death Dis. 2018. PMID: 29500439 Free PMC article.
-
Strategies to overcome DC dysregulation in the tumor microenvironment.Front Immunol. 2022 Oct 6;13:980709. doi: 10.3389/fimmu.2022.980709. eCollection 2022. Front Immunol. 2022. PMID: 36275666 Free PMC article. Review.
-
Induction of type I and type III interferons by Borrelia burgdorferi correlates with pathogenesis and requires linear plasmid 36.PLoS One. 2014 Jun 19;9(6):e100174. doi: 10.1371/journal.pone.0100174. eCollection 2014. PLoS One. 2014. PMID: 24945497 Free PMC article.
-
Identifying a role for Toll-like receptor 3 in the innate immune response to Chlamydia muridarum infection in murine oviduct epithelial cells.Infect Immun. 2012 Jan;80(1):254-65. doi: 10.1128/IAI.05549-11. Epub 2011 Oct 17. Infect Immun. 2012. PMID: 22006569 Free PMC article.
-
A high-resolution view of the immune and stromal cell response to Haemophilus ducreyi infection in human volunteers.mBio. 2025 Mar 12;16(3):e0388524. doi: 10.1128/mbio.03885-24. Epub 2025 Jan 30. mBio. 2025. PMID: 39882906 Free PMC article.
References
-
- Adams O., et al. 2004. Inhibition of human herpes simplex virus type 2 by interferon gamma and tumor necrosis factor alpha is mediated by indoleamine 2,3-dioxygenase. Microbes Infect. 6:806–812 - PubMed
-
- Baban B., et al. 2005. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. Int. Immunol. 17:909–919 - PubMed
-
- Babcock T. A., Carlin J. M. 2000. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells. Cytokine 12:588–594 - PubMed
-
- Banks K. E., et al. 2008. The enterobacterial common antigen-like gene cluster of Haemophilus ducreyi contributes to virulence in humans. J. Infect. Dis. 197:1531–1536 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

