Genomic and surface proteomic analysis of the canine pathogen Staphylococcus pseudintermedius reveals proteins that mediate adherence to the extracellular matrix
- PMID: 21576333
- PMCID: PMC3147560
- DOI: 10.1128/IAI.00137-11
Genomic and surface proteomic analysis of the canine pathogen Staphylococcus pseudintermedius reveals proteins that mediate adherence to the extracellular matrix
Abstract
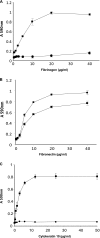
Cell wall-associated (CWA) proteins made by Gram-positive pathogens play a fundamental role in pathogenesis. Staphylococcus pseudintermedius is a major animal pathogen responsible for the canine skin disease bacterial pyoderma. Here, we describe the bioinformatic analysis of the family of 18 predicted CWA proteins encoded in the genome of S. pseudintermedius strain ED99 and determine their distribution among a phylogenetically diverse panel of S. pseudintermedius clinical isolates and closely related species of the Staphylococcus intermedius group. In parallel, we employed a proteomic approach to identify proteins presented on the surface of strain ED99 in vitro, revealing a total of 60 surface-localized proteins in one or more phases of growth, including 6 of the 18 genome-predicted CWA proteins. Based on these analyses, we selected two CWA proteins (SpsD and SpsL) encoded by all strains examined and investigated their capacity to mediate adherence to extracellular matrix proteins. We discovered that SpsD and SpsL mediated binding of a heterologous host, Lactococcus lactis, to fibrinogen and fibronectin and that SpsD mediated binding to cytokeratin 10, a major constituent of mammalian skin. Of note, the interaction with fibrinogen was host-species dependent, suggestive of a role for SpsD and SpsL in the host tropism of S. pseudintermedius. Finally, we identified IgG specific for SpsD and SpsL in sera from dogs with bacterial pyoderma, implying that both proteins are expressed during infection. The combined genomic and proteomic approach employed in the current study has revealed novel host-pathogen interactions which represent candidate therapeutic targets for the control of bacterial pyoderma.
Figures




References
-
- Batycka M., et al. 2006. Ultra-fast tandem mass spectrometry scanning combined with monolithic column liquid chromatography increase throughput in proteomic analysis. - PubMed
-
- Carneiro C. R., Postol E., Nomizo R., Reis L. F., Brentani R. R. 2004. Identification of enolase as a laminin-binding protein on the surface of Staphylococcus aureus. Microbes Infect. 6:604–608 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

