IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease
- PMID: 21576383
- PMCID: PMC3173242
- DOI: 10.1084/jem.20101712
IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease
Abstract
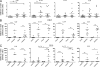
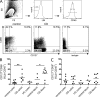
Results of experimental and genetic studies have highlighted the role of the IL-23/IL-17 axis in the pathogenesis of inflammatory bowel disease (IBD). IL-23-driven inflammation has been primarily linked to Th17 cells; however, we have recently identified a novel population of innate lymphoid cells (ILCs) in mice that produces IL-17, IL-22, and IFN-γ in response to IL-23 and mediates innate colitis. The relevance of ILC populations in human health and disease is currently poorly understood. In this study, we have analyzed the role of IL-23-responsive ILCs in the human intestine in control and IBD patients. Our results show increased expression of the Th17-associated cytokine genes IL17A and IL17F among intestinal CD3⁻ cells in IBD. IL17A and IL17F expression is restricted to CD56⁻ ILCs, whereas IL-23 induces IL22 and IL26 in the CD56⁺ ILC compartment. Furthermore, we observed a significant and selective increase in CD127⁺CD56⁻ ILCs in the inflamed intestine in Crohn's disease (CD) patients but not in ulcerative colitis patients. These results indicate that IL-23-responsive ILCs are present in the human intestine and that intestinal inflammation in CD is associated with the selective accumulation of a phenotypically distinct ILC population characterized by inflammatory cytokine expression. ILCs may contribute to intestinal inflammation through cytokine production, lymphocyte recruitment, and organization of the inflammatory tissue and may represent a novel tissue-specific target for subtypes of IBD.
Figures

References
-
- Andoh A., Zhang Z., Inatomi O., Fujino S., Deguchi Y., Araki Y., Tsujikawa T., Kitoh K., Kim-Mitsuyama S., Takayanagi A., et al. 2005. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 129:969–984 10.1053/j.gastro.2005.06.071 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

