Lipid phosphate phosphatase 3 enables efficient thymic egress
- PMID: 21576386
- PMCID: PMC3173249
- DOI: 10.1084/jem.20102551
Lipid phosphate phosphatase 3 enables efficient thymic egress
Abstract
The signaling lipid sphingosine-1-phosphate (S1P) stabilizes the vasculature, directs lymphocyte egress from lymphoid organs, and shapes inflammatory responses. However, little is known about how S1P distribution is controlled in vivo, and it is not clear how a ubiquitously made lipid functions as a signal that requires precise spatial and temporal control. We have found that lipid phosphate phosphatase 3 (LPP3) enables efficient export of mature T cells from the thymus into circulation, and several lines of evidence suggest that LPP3 promotes exit by destroying thymic S1P. Although five additional S1P-degrading enzymes are expressed in the thymus, they cannot compensate for the loss of LPP3. Moreover, conditional deletion of LPP3 in either epithelial cells or endothelial cells is sufficient to inhibit egress. These results suggest that S1P generation and destruction are tightly regulated and that LPP3 is essential to establish the balance.
Figures

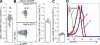

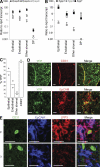
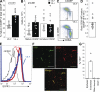
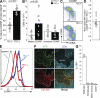
References
-
- Barilà D., Plateroti M., Nobili F., Muda A.O., Xie Y., Morimoto T., Perozzi G. 1996. The Dri 42 gene, whose expression is up-regulated during epithelial differentiation, encodes a novel endoplasmic reticulum resident transmembrane protein. J. Biol. Chem. 271:29928–29936 10.1074/jbc.271.47.29928 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

