Lymphatic vascular morphogenesis in development, physiology, and disease
- PMID: 21576390
- PMCID: PMC3166860
- DOI: 10.1083/jcb.201012094
Lymphatic vascular morphogenesis in development, physiology, and disease
Abstract
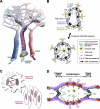
The lymphatic vasculature constitutes a highly specialized part of the vascular system that is essential for the maintenance of interstitial fluid balance, uptake of dietary fat, and immune response. Recently, there has been an increased awareness of the importance of lymphatic vessels in many common pathological conditions, such as tumor cell dissemination and chronic inflammation. Studies of embryonic development and genetically engineered animal models coupled with the discovery of mutations underlying human lymphedema syndromes have contributed to our understanding of mechanisms regulating normal and pathological lymphatic morphogenesis. It is now crucial to use this knowledge for the development of novel therapies for human diseases.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

