The conversion of centrioles to centrosomes: essential coupling of duplication with segregation
- PMID: 21576395
- PMCID: PMC3166877
- DOI: 10.1083/jcb.201101109
The conversion of centrioles to centrosomes: essential coupling of duplication with segregation
Abstract
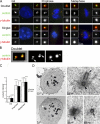
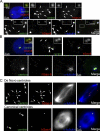
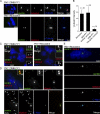
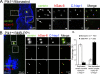
Centrioles are self-reproducing organelles that form the core structure of centrosomes or microtubule-organizing centers (MTOCs). However, whether duplication and MTOC organization reflect innate activities of centrioles or activities acquired conditionally is unclear. In this paper, we show that newly formed full-length centrioles had no inherent capacity to duplicate or to organize pericentriolar material (PCM) but acquired both after mitosis through a Plk1-dependent modification that occurred in early mitosis. Modified centrioles initiated PCM recruitment in G1 and segregated equally in mitosis through association with spindle poles. Conversely, unmodified centrioles segregated randomly unless passively tethered to modified centrioles. Strikingly, duplication occurred only in centrioles that were both modified and disengaged, whereas unmodified centrioles, engaged or not, were "infertile," indicating that engagement specifically blocks modified centrioles from reduplication. These two requirements, centriole modification and disengagement, fully exclude unlimited duplication in one cell cycle. We thus uncovered a Plk1-dependent mechanism whereby duplication and segregation are coupled to maintain centriole homeostasis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

