Joint population pharmacokinetic analysis of zidovudine, lamivudine, and their active intracellular metabolites in HIV patients
- PMID: 21576446
- PMCID: PMC3122424
- DOI: 10.1128/AAC.01487-10
Joint population pharmacokinetic analysis of zidovudine, lamivudine, and their active intracellular metabolites in HIV patients
Abstract
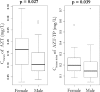
The population pharmacokinetic parameters of zidovudine (AZT), lamivudine (3TC), and their active intracellular metabolites in 75 naïve HIV-infected patients receiving an oral combination of AZT and 3TC twice daily as part of their multitherapy treatment in the COPHAR2-ANRS 111 trial are described. Four blood samples per patient were taken after 2 weeks of treatment to measure drug concentrations at steady state. Plasma AZT and 3TC concentrations were measured in 73 patients, and among those, 62 patients had measurable intracellular AZT-TP and 3TC-TP concentrations. For each drug, a joint population pharmacokinetic model was developed and we investigated the influence of different covariates. We then studied correlations between the mean plasma and intracellular concentrations of each drug. A one-compartment model with first-order absorption and elimination best described the plasma AZT concentration, with an additional compartment for intracellular AZT-TP. A similar model but with zero-order absorption was found to adequately described concentrations of 3TC and its metabolite 3TC-TP. The half-lives of AZT and 3TC were 0.81 h (94.8%) and 2.97 h (39.2%), respectively, whereas the intracellular half-lives of AZT-TP and 3TC-TP were 10.73 h (69%) and 21.16 h (44%), respectively. We found particularly a gender effect on the apparent bioavailability of AZT, as well as on the mean plasma and intracellular concentrations of AZT, which were significantly higher in females than in males. Relationships between mean plasma drug and intracellular metabolite concentrations were also highlighted both for AZT and for 3TC. Simulation with the model of plasma and intracellular concentrations for once- versus twice-daily regimens suggested that a daily dosing regimen with double doses could be appropriate.
Figures
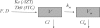



References
-
- Anderson P. L., Kakuda T. N., Kawle S., Fletcher C. V. 2003. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS 17:2159–2168 - PubMed
-
- Angel J. B., et al. 1993. Pharmacokinetics of 3TC (GR109714X) administered with and without food to HIV-infected patients. Clin. Drug Investig. 6:70–74
-
- Aweeka F. T., et al. 2007. Pharmacokinetic evaluation of the effects of ribavirin on zidovudine triphosphate formation: ACTG 5092s Study Team. HIV Med. 8:288–294 - PubMed
-
- Aweeka F. T., et al. 2006. The impact of sex and contraceptive therapy on the plasma and intracellular pharmacokinetics of zidovudine. AIDS 20:1833–1841 - PubMed
-
- Barry M. G., et al. 1996. The effect of zidovudine dose on the formation of intracellular phosphorylated metabolites. AIDS 10:1361–1367 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

